Islet autoantibodies as precision diagnostic tools to characterize heterogeneity in type 1 diabetes: a systematic review
- PMID: 38582818
- PMCID: PMC10998887
- DOI: 10.1038/s43856-024-00478-y
Islet autoantibodies as precision diagnostic tools to characterize heterogeneity in type 1 diabetes: a systematic review
Abstract
Background: Islet autoantibodies form the foundation for type 1 diabetes (T1D) diagnosis and staging, but heterogeneity exists in T1D development and presentation. We hypothesized that autoantibodies can identify heterogeneity before, at, and after T1D diagnosis, and in response to disease-modifying therapies.
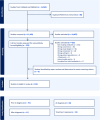
Methods: We systematically reviewed PubMed and EMBASE databases (6/14/2022) assessing 10 years of original research examining relationships between autoantibodies and heterogeneity before, at, after diagnosis, and in response to disease-modifying therapies in individuals at-risk or within 1 year of T1D diagnosis. A critical appraisal checklist tool for cohort studies was modified and used for risk of bias assessment.
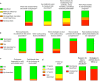
Results: Here we show that 152 studies that met extraction criteria most commonly characterized heterogeneity before diagnosis (91/152). Autoantibody type/target was most frequently examined, followed by autoantibody number. Recurring themes included correlations of autoantibody number, type, and titers with progression, differing phenotypes based on order of autoantibody seroconversion, and interactions with age and genetics. Only 44% specifically described autoantibody assay standardization program participation.
Conclusions: Current evidence most strongly supports the application of autoantibody features to more precisely define T1D before diagnosis. Our findings support continued use of pre-clinical staging paradigms based on autoantibody number and suggest that additional autoantibody features, particularly in relation to age and genetic risk, could offer more precise stratification. To improve reproducibility and applicability of autoantibody-based precision medicine in T1D, we propose a methods checklist for islet autoantibody-based manuscripts which includes use of precision medicine MeSH terms and participation in autoantibody standardization workshops.
Plain language summary
Islet autoantibodies are markers found in the blood when insulin-producing cells in the pancreas become damaged and can be used to predict future development of type 1 diabetes. We evaluated published literature to determine whether characteristics of islet antibodies (type, levels, numbers) could improve prediction and help understand differences in how individuals with type 1 diabetes respond to treatments. We found existing evidence shows that islet autoantibody type and number are most useful to predict disease progression before diagnosis. In addition, the age when islet autoantibodies first appear strongly influences rate of progression. These findings provide important information for patients and care providers on how islet autoantibodies can be used to understand future type 1 diabetes development and to identify individuals who have the potential to benefit from intervention or prevention therapy.
© 2024. The Author(s).
Conflict of interest statement
E.K.S. has received compensation for educational lectures from Medscape, ADA, and MJH Life Sciences and as a consultant for DRI Healthcare. C.E.M. reported serving on advisory boards for Provention Bio, Isla Technologies, MaiCell Technologies, Avotres, DiogenyX, and Neurodon; receiving in-kind research support from Bristol Myers Squibb and Nimbus Pharmaceuticals; and receiving investigator initiated grants from Lilly Pharmaceuticals and Astellas Pharmaceuticals. L.A.D. reports research support to institution from Dompe, Lilly, Mannkind, Provention, Zealand and consulting relationships with Abata and Vertex. R.A.O. had a UK MRC Confidence in concept grant to develop a T1D GRS biochip with Randox Ltd, and has ongoing research funding from Randox R&D. No other authors report any relevant conflicts of interest.
Figures

References
Grants and funding
- U01 DK127786/DK/NIDDK NIH HHS/United States
- UC4 DK117483/DK/NIDDK NIH HHS/United States
- UL1 TR001108/TR/NCATS NIH HHS/United States
- R01 CA231226/CA/NCI NIH HHS/United States
- K12 DK133995/DK/NIDDK NIH HHS/United States
- R01 DK122586/DK/NIDDK NIH HHS/United States
- R01 DK093954/DK/NIDDK NIH HHS/United States
- R01 DK133881/DK/NIDDK NIH HHS/United States
- R01 DK121929/DK/NIDDK NIH HHS/United States
- R01 DK127308/DK/NIDDK NIH HHS/United States
- R01 HL149676/HL/NHLBI NIH HHS/United States
- UC4 DK104166/DK/NIDDK NIH HHS/United States
- R01 DK121843/DK/NIDDK NIH HHS/United States
- I01 BX001733/BX/BLRD VA/United States
- R01 DK124395/DK/NIDDK NIH HHS/United States
- R01 AI141952/AI/NIAID NIH HHS/United States
- R01 DK127236/DK/NIDDK NIH HHS/United States
- R03 DK127472/DK/NIDDK NIH HHS/United States
- U54 DK118638/DK/NIDDK NIH HHS/United States
- U01 DK127382/DK/NIDDK NIH HHS/United States
- P30 DK097512/DK/NIDDK NIH HHS/United States
- K23 DK129799/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

