Pharmacokinetics of Monoclonal Antibodies Throughout Pregnancy: A Systematic Literature Review
- PMID: 38583128
- PMCID: PMC11106164
- DOI: 10.1007/s40262-024-01370-7
Pharmacokinetics of Monoclonal Antibodies Throughout Pregnancy: A Systematic Literature Review
Abstract
Background and objective: Although little information is available on the pharmacokinetics (PK) of monoclonal antibodies (mAbs) during pregnancy, multiple mAbs are being used during pregnancy for various indications. The aim of this systematic literature review was to characterize the PK of mAbs throughout pregnancy.
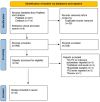
Methods: A systematic literature search was carried out in PubMed and Embase on 21 April 2023. Articles were included when information on PK or exposure parameters of mAbs in pregnant women was available.
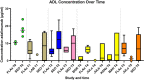
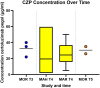
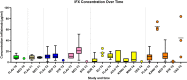
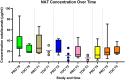
Results: A total of 42 relevant articles were included, of which eight discussed adalimumab, three certolizumab pegol, five eculizumab, one golimumab, 12 infliximab (IFX), two natalizumab, one canakinumab, one omalizumab, five tocilizumab, eight ustekinumab, and five vedolizumab. One of the 42 studies reported information on clearance (CL) and volume of distribution (VD) of IFX; all other studies only reported on serum concentrations in the pre-pregnancy state, different trimesters, and the postpartum period. For all of the assessed mAbs except IFX, serum concentrations were similar to concentrations in the pre-pregnancy state or modestly decreased. In contrast, IFX trough concentrations generally increased in the second and third trimesters in comparison to the non-pregnant state.
Conclusion: Available information suggests that the anatomical and physiological changes throughout pregnancy may have meaningful effects on the PK of mAbs. For most mAbs (not IFX), modestly higher dosing (per mg) maybe needed during pregnancy to sustain a similar serum exposure compared to pre-pregnancy.
© 2024. The Author(s).
Conflict of interest statement
This research did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors. Competing interests: Jip van Gendt, Robin Emaus, Marijn C. Visschedijk, Dianne Bouwknegt, Karina de Leeuw, Jelmer R. Prins, and Paola Mian declare that they have no financial or non-financial interests that are directly or indirectly related to the work submitted for publication. Daan J. Touw is vice chair of the Medical Advisory Board of Sanquin. Paul Malik is a full-time employee of Calico Life Sciences.
Figures


References
-
- European Medicines Agency. Humira | European Medicines Agency. European Medicines Agency, 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/humira (accessed Jul. 25, 2023).
-
- European Medicines Agency. Cimzia | European Medicines Agency. European Medicines Agency, 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/cimzia (accessed Jul. 25, 2023).
-
- European Medicines Agency. Simponi | European Medicines Agency. European Medicine Agency, 2019. https://www.ema.europa.eu/en/medicines/human/EPAR/simponi (accessed Jul. 25, 2023).
-
- European Medicines Agency. Remicade | European Medicines Agency. European Medicines Agency, 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/remicade (accessed Jul. 25, 2023).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

