Parabrachial Calca neurons drive nociplasticity
- PMID: 38583149
- PMCID: PMC11210282
- DOI: 10.1016/j.celrep.2024.114057
Parabrachial Calca neurons drive nociplasticity
Abstract
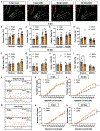
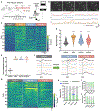
Pain that persists beyond the time required for tissue healing and pain that arises in the absence of tissue injury, collectively referred to as nociplastic pain, are poorly understood phenomena mediated by plasticity within the central nervous system. The parabrachial nucleus (PBN) is a hub that relays aversive sensory information and appears to play a role in nociplasticity. Here, by preventing PBN Calca neurons from releasing neurotransmitters, we demonstrate that activation of Calca neurons is necessary for the manifestation and maintenance of chronic pain. Additionally, by directly stimulating Calca neurons, we demonstrate that Calca neuron activity is sufficient to drive nociplasticity. Aversive stimuli of multiple sensory modalities, such as exposure to nitroglycerin, cisplatin, or lithium chloride, can drive nociplasticity in a Calca-neuron-dependent manner. Aversive events drive nociplasticity in Calca neurons in the form of increased activity and excitability; however, neuroplasticity also appears to occur in downstream circuitry.
Keywords: CP: Cell biology; CP: Neuroscience; Calca; allodynia; calcitonin gene-related peptide (CGRP); calcium imaging; cisplatin; mechanical sensitivity (Von Frey assay); neuroplasticity; nitroglycerin.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





Update of
-
Parabrachial Calca neurons drive nociplasticity.bioRxiv [Preprint]. 2023 Oct 31:2023.10.26.564223. doi: 10.1101/2023.10.26.564223. bioRxiv. 2023. Update in: Cell Rep. 2024 Apr 23;43(4):114057. doi: 10.1016/j.celrep.2024.114057. PMID: 37961621 Free PMC article. Updated. Preprint.
References
-
- Nijs J, Lahousse A, Kapreli E, Bilika P, Saraçoğlu I, Malfliet A, Coppieters I, De Baets L, Leysen L, Roose E, et al. (2021). Nociplastic pain criteria or recognition of central sensitization? Pain phenotyping in the past, present and future. J. Clin. Med 10, 3203. 10.3390/jcm10153203. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

