Clinical Benefit and Regulatory Outcomes of Cancer Drugs Receiving Accelerated Approval
- PMID: 38583175
- PMCID: PMC11000139
- DOI: 10.1001/jama.2024.2396
Clinical Benefit and Regulatory Outcomes of Cancer Drugs Receiving Accelerated Approval
Abstract
Importance: The US Food and Drug Administration's (FDA) accelerated approval pathway allows approval of investigational drugs treating unmet medical needs based on changes to surrogate measures considered "reasonably likely" to predict clinical benefit. Postapproval clinical trials are then required to confirm whether these drugs offer clinical benefit.
Objective: To determine whether cancer drugs granted accelerated approval ultimately demonstrate clinical benefit and to evaluate the basis of conversion to regular approval.
Design, setting, and participants: In this cohort study, publicly available FDA data were used to identify cancer drugs granted accelerated approval from 2013 to 2023.
Main outcomes and measures: Demonstrated improvement in quality of life or overall survival in accelerated approvals with more than 5 years of follow-up, as well as confirmatory trial end points and time to conversion for drug-indication pairs converted to regular approval.
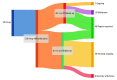
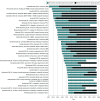
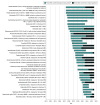
Results: A total of 129 cancer drug-indication pairs were granted accelerated approval from 2013 to 2023. Among 46 indications with more than 5 years of follow-up (approved 2013-2017), approximately two-thirds (29, 63%) were converted to regular approval, 10 (22%) were withdrawn, and 7 (15%) remained ongoing after a median of 6.3 years. Fewer than half (20/46, 43%) demonstrated a clinical benefit in confirmatory trials. Time to withdrawal decreased from 9.9 years to 3.6 years, and time to regular approval increased from 1.6 years to 3.6 years. Among 48 drug-indication pairs converted to regular approval, 19 (40%) were converted based on overall survival, 21 (44%) on progression-free survival, 5 (10%) on response rate plus duration of response, 2 (4%) on response rate, and 1 (2%) despite a negative confirmatory trial. Comparing accelerated and regular approval indications, 18 of 48 (38%) were unchanged, while 30 of 48 (63%) had different indications (eg, earlier line of therapy).
Conclusions and relevance: Most cancer drugs granted accelerated approval did not demonstrate benefit in overall survival or quality of life within 5 years of accelerated approval. Patients should be clearly informed about the cancer drugs that use the accelerated approval pathway and do not end up showing benefits in patient-centered clinical outcomes.
Conflict of interest statement
Figures
References
-
- US Food and Drug Administration . Applications for FDA approval to market a new drug: subpart A: general provisions: purpose. 21 CFR §314.2 (2023).
-
- US Food and Drug Administration . Accelerated Approval Program. Accessed August 21, 2023. https://www.fda.gov/drugs/nda-and-bla-approvals/accelerated-approval-pro...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

