Engineered extracellular vesicle-based gene therapy for the treatment of discogenic back pain
- PMID: 38583365
- PMCID: PMC11164054
- DOI: 10.1016/j.biomaterials.2024.122562
Engineered extracellular vesicle-based gene therapy for the treatment of discogenic back pain
Abstract
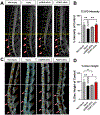
Painful musculoskeletal disorders such as intervertebral disc (IVD) degeneration associated with chronic low back pain (termed "Discogenic back pain", DBP), are a significant socio-economic burden worldwide and contribute to the growing opioid crisis. Yet there are very few if any successful interventions that can restore the tissue's structure and function while also addressing the symptomatic pain. Here we have developed a novel non-viral gene therapy, using engineered extracellular vesicles (eEVs) to deliver the developmental transcription factor FOXF1 to the degenerated IVD in an in vivo model. Injured IVDs treated with eEVs loaded with FOXF1 demonstrated robust sex-specific reductions in pain behaviors compared to control groups. Furthermore, significant restoration of IVD structure and function in animals treated with FOXF1 eEVs were observed, with significant increases in disc height, tissue hydration, proteoglycan content, and mechanical properties. This is the first study to successfully restore tissue function while modulating pain behaviors in an animal model of DBP using eEV-based non-viral delivery of transcription factor genes. Such a strategy can be readily translated to other painful musculoskeletal disorders.
Keywords: Cell reprogramming; Engineered extracellular vesicles; Intervertebral disc; Low back pain; Nanocarriers; Non-viral gene delivery.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Schwarzer AC, Aprill CN, Derby R, Fortin J, Kine G, Bogduk N, The prevalence and clinical features of internal disc disruption in patients with chronic low back pain, Spine 20 (1995) 1878–1883. - PubMed
-
- Luoma K, Riihimäki H, Luukkonen R, Raininko R, Viikari-Juntura E, Lamminen A, Low back pain in relation to lumbar disc degeneration, Spine 25 (2000) 487–492. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

