The Lancet Commission on prostate cancer: planning for the surge in cases
- PMID: 38583453
- PMCID: PMC7617369
- DOI: 10.1016/S0140-6736(24)00651-2
The Lancet Commission on prostate cancer: planning for the surge in cases
Erratum in
-
Department of Error.Lancet. 2024 Apr 27;403(10437):1634. doi: 10.1016/S0140-6736(24)00748-7. Epub 2024 Apr 10. Lancet. 2024. PMID: 38614115 No abstract available.
Abstract
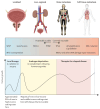
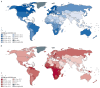
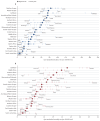
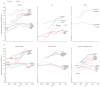
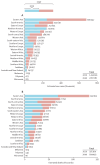
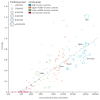
Prostate cancer is the most common cancer in men in 112 countries, and accounts for 15% of cancers. In this Commission, we report projections of prostate cancer cases in 2040 on the basis of data for demographic changes worldwide and rising life expectancy. Our findings suggest that the number of new cases annually will rise from 1·4 million in 2020 to 2·9 million by 2040. This surge in cases cannot be prevented by lifestyle changes or public health interventions alone, and governments need to prepare strategies to deal with it. We have projected trends in the incidence of prostate cancer and related mortality (assuming no changes in treatment) in the next 10–15 years, and make recommendations on how to deal with these issues.
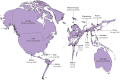
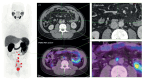
For the Commission, we established four working groups, each of which examined a different aspect of prostate cancer: epidemiology and future projected trends in cases, the diagnostic pathway, treatment, and management of advanced disease, the main problem for most men diagnosed with prostate cancer worldwide. Throughout we have separated problems in high-income countries (HICs) from those in low-income and middle-income countries (LMICs), although we acknowledge that this distinction can be an oversimplification (some rich patients in LMICs can access high-quality care, whereas many patients in HICs, especially the USA, cannot because of inadequate insurance coverage). The burden of disease globally is already substantial, but options to improve care are already available at moderate cost. We found that late diagnosis is widespread worldwide, but especially in LMICs, where it is the norm. Early diagnosis improves prognosis and outcomes, and reduces societal and individual costs, and we recommend changes to the diagnostic pathway that can be immediately implemented. For men diagnosed with advanced disease, optimal use of available technologies, adjusted to the resource levels available, could produce improved outcomes. We also found that demographic changes (ie, changing age structures and increasing life expectancy) in LMICs will drive big increases in prostate cancer, and cases are also projected to rise in high-income countries. This projected rise in cases has driven the main thrust of our recommendations throughout. Dealing with this rise in cases will require urgent and radical interventions, particularly in LMICs, including an emphasis on education (both of health professionals and the general population) linked to outreach programmes to increase awareness. If implemented, these interventions would shift the case mix from advanced to earlier-stage disease, which in turn would necessitate different treatment approaches: earlier diagnosis would prompt a shift from palliative to curative therapies based around surgery and radiotherapy. Although age-adjusted mortality from prostate cancer is falling in HICs, it is rising in LMICs. And, despite large, well known differences in disease incidence and mortality by ethnicity (eg, incidence in men of African heritage is roughly double that in men of European heritage), most prostate cancer research has disproportionally focused on men of European heritage. Without urgent action, these trends will cause global deaths from prostate cancer to rise rapidly.
Conflict of interest statement
Declaration of interests NDJ reports advisory board and personal fees from AstraZeneca, Bayer, Clovis, Janssen, Merck, Merck Sharp & Dohme, Novartis, Sanofi, Astellas, and AAA Accelerator Solutions. FF reports personal fees from Janssen, Astellas, Serimmune, Foundation Medicine, Exact Sciences, Bristol-Myers Squibb, Varian Medical Systems, Novartis, Roivant, Myovant, Bayer, BlueStar Genomics, Artera, Tempus, Genentech, PFS Genomics, and Amgen, and holds stock options in Serimmune, BlueStar Genomics, and Artera. SG reports fees from Tolremo, Ipsen, Silvio Grasso Consulting, WebMD–Medscape, the American Society of Clinical Oncology, European Society for Medical Oncology, Peer Voice, SAKK, the German-speaking European School of Oncology, Radiotelevisione Svizzera Italiana, the Swiss Academy of Multidisciplinary Oncology, Meister ConCept, AdMeTech Foundation, EPG Health, Intellisphere, and Schweizerische Gessellschaft für Medizineische Onkologie. SG also reports travel support from AstraZeneca, Bayer, Intellisphere, and Gilead, paid advisory board participation for Merck Sharp & Dohme, Telixpharma, Bristol-Myers Squibb, AAA International, Orion, Bayer, Novartis, Modra Pharmaceuticals, AstraZeneca, Myriad Genetic, Daiichi Sankyo, Boehringer Ingelheim, Innomedica, Macrogenics, and Pfizer, and holds a patent (WO2009138392). BA-L reports fees from the Cancer Prevention and Research Institute of Texas, the Commonwealth Foundation, the Lyda Hill Foundation, Cancer Research UK, the Wellcome Trust, the Bob Champion Trust, AstraZeneca, Astex Pharmaceuticals, the New York Genome Center, and Existentia, travel support from Cancer Research UK, Astex, the STAT summit, the American Society of Hematology, and the American Association for Cancer Research, and participation in a Cancer Research UK data strategy board. GA reports fees from Janssen, Novartis, Astellas, the Institute of Cancer Research, Veracyte, Artera, Pfizer, AstraZeneca, Astellas, Novartis, Arvinas, Bayer, Sanofi, Propella, and Orion, holds a patent related to blood-based methylation markers (GB1915469.9), and has received equipment from Agilent. EC reports fees from Janssen. RE reports book royalties plus support and fees from the UK National Institute for Health and Care Research, AstraZeneca, Bayer, Ipsen, the Active Surveillance Movember Committee, the American Society of Clinical Oncology, University of Chicago, Dana Farber Cancer Institute, the Spanish National Cancer Research Center, Our Future Health, Jnetics UK, the Institute of Cancer Research, and Convergence Science Centre. RE also reports a pending Cancer Research UK patent, a stock ISA, receipt of gifts from patients (within limits allowed), and other financial interests in private medical practice. SH reports participation on data safety monitoring boards and advisory boards. DH reports fees from Techtrials, Astellas, Adium, Ipsen, Janssen, Bayer, Merck Sharp & Dohme, and Pfizer. MSH reports fees or grant funding from the Prostate Cancer Foundation, the Prostate Cancer Theranostics and Imaging Centre of Excellence, the Australian National Health and Medical Research Council, Movember, the US Department of Defense, Medical Research Future Fund, Bayer, the Peter MacCallum Foundation, Isotopia, the Australian Nuclear Science and Technology Organisation, Merck Sharpe & Dohme, Novartis, AstraZeneca, and Astellas. MSH also reports unrenumerated leadership or fiduciary role in Australian Friends of Sheba. MMog reports fees from NHS England, the UK National Institute for Health and Care Research, and Bayer. CM reports fees from UK National Institute for Health and Care Research, the UK Medical Research Council, Prostate Cancer UK, Cancer Rsearch UK, Sonacare, Ipsen, Bayer, and Astellas. AMo reports fees from Bayer, Myovant, Pfizer, Astellas, AstraZeneca, AAA, Bayer, Exelixis, Janssen, Lantheus, Myovant, Merck, Novartis, Sanofi, and Telix, participation in data Safety monitoring boards and advisory boards for Gilead, and a leadership or fiduciary role in ZERO Prostate Cancer. MMor reports fees from the National Cancer Institute Comprehensive Cancer Center, Lantheus, AstraZeneca, Amgen, Daiichi, Convergent, Pfizer, Clarity, Blue Earth Diagnostics, POINT Diagnostics, Z-Alpha, Ambrx, Flare, Fusion, Curium, Transtherabio, Doximity, BMS, and Celgene, reports a US patent application (18/448 609) for a method of treating prostate cancer, and holds stock options in Doximity. DM reports fees from Novartis, Janssen, Bayer, Astellas, Ipsen, and AstraZeneca. PLN reports fees from Bayer, Astellas, Boston Scientific, AIQ, Astellas, Novartis, Janssen, Blue Earth, Nanocan, and Theranano, and holds stock options in Stratagen Bio, Nanocan, and Reversal Therapeutics. CP reports fees from Artera, which has a financial relationship with University College London (his employer) as part of a data licensing agreement. All other authors declare no competing interests.
Figures

Comment in
-
Offline: It is time to take cancer more seriously.Lancet. 2024 Apr 27;403(10437):1616. doi: 10.1016/S0140-6736(24)00853-5. Lancet. 2024. PMID: 38677847 No abstract available.
References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. - PubMed
-
- Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: cancer today. 2020. [accessed Jan 22, 2023]. https://gco.iarc.fr/today .
-
- Bray F, Colombet M, Aitken JF, et al., editors. Cancer incidence in five continents, vol XII (IARC CancerBase No 19) 2023. [accessed Nov 10, 2023]. https://ci5.iarc.who.int/ci5-xii .
-
- Sandhu S, Moore CM, Chiong E, Beltran H, Bristow RG, Williams SG. Prostate cancer. Lancet. 2021;398:1075–90. - PubMed
-
- Culp MB, Soerjomataram I, Efstathiou JA, Bray F, Jemal A. Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol. 2020;77:38–52. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

