Timing of radiotherapy (RT) after radical prostatectomy (RP): long-term outcomes in the RADICALS-RT trial (NCT00541047)
- PMID: 38583574
- PMCID: PMC7617161
- DOI: 10.1016/j.annonc.2024.03.010
Timing of radiotherapy (RT) after radical prostatectomy (RP): long-term outcomes in the RADICALS-RT trial (NCT00541047)
Abstract
Background: The optimal timing of radiotherapy (RT) after radical prostatectomy for prostate cancer has been uncertain. RADICALS-RT compared efficacy and safety of adjuvant RT versus an observation policy with salvage RT for prostate-specific antigen (PSA) failure.
Patients and methods: RADICALS-RT was a randomised controlled trial enrolling patients with ≥1 risk factor (pT3/4, Gleason 7-10, positive margins, preoperative PSA≥10 ng/ml) for recurrence after radical prostatectomy. Patients were randomised 1:1 to adjuvant RT ('Adjuvant-RT') or an observation policy with salvage RT for PSA failure ('Salvage-RT') defined as PSA≥0.1 ng/ml or three consecutive rises. Stratification factors were Gleason score, margin status, planned RT schedule (52.5 Gy/20 fractions or 66 Gy/33 fractions) and treatment centre. The primary outcome measure was freedom-from-distant-metastasis (FFDM), designed with 80% power to detect an improvement from 90% with Salvage-RT (control) to 95% at 10 years with Adjuvant-RT. Secondary outcome measures were biochemical progression-free survival, freedom from non-protocol hormone therapy, safety and patient-reported outcomes. Standard survival analysis methods were used; hazard ratio (HR)<1 favours Adjuvant-RT.
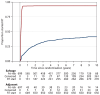
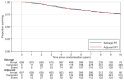
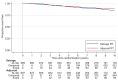
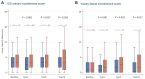
Results: Between October 2007 and December 2016, 1396 participants from UK, Denmark, Canada and Ireland were randomised: 699 Salvage-RT, 697 Adjuvant-RT. Allocated groups were balanced with a median age of 65 years. Ninety-three percent (649/697) Adjuvant-RT reported RT within 6 months after randomisation; 39% (270/699) Salvage-RT reported RT during follow-up. Median follow-up was 7.8 years. With 80 distant metastasis events, 10-year FFDM was 93% for Adjuvant-RT and 90% for Salvage-RT: HR=0.68 [95% confidence interval (CI) 0.43-1.07, P=0.095]. Of 109 deaths, 17 were due to prostate cancer. Overall survival was not improved (HR=0.980, 95% CI 0.667-1.440, P=0.917). Adjuvant-RT reported worse urinary and faecal incontinence 1 year after randomisation (P=0.001); faecal incontinence remained significant after 10 years (P=0.017).
Conclusion: Long-term results from RADICALS-RT confirm adjuvant RT after radical prostatectomy increases the risk of urinary and bowel morbidity, but does not meaningfully improve disease control. An observation policy with salvage RT for PSA failure should be the current standard after radical prostatectomy.
Trial identification: RADICALS, RADICALS-RT, ISRCTN40814031, NCT00541047.
Keywords: clinical trial; long-term follow-up; observational; prostate cancer; radiotherapy; randomised controlled trial.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
CCP reports advisory board for AAA and Blue Earth Therapeutics and ITM Oncologics. PMP reports personal fees for participation on advisory board for AAA Nordic, MSD, Pfizer Denmark and Bayer A/S Denmark. CC reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Bayer Corp, Knight Therapeutics, and AbbVie. NDJ reports personal fees for participation on advisory board for AstraZeneca, Bayer, Janssen, Merck, Novartis and Sanofi; institutional fees for Expert Testimony for Janssen and Sanofi; personal fees for being an Invited Speaker for Merck Sharp & Dohme (UK) Limited. FS reports advisory board for Astellas, AstraZeneca, Bayer, BMS, Janssen, Merck, Myovant, Novartis and Pfizer; local PI for Amgen, Astellas, Bayer, BMS, Janssen, Merck, Novartis, Pfizer and Sanofi; coordinating PI for AstraZeneca. LB reports a previous role, unrelated to the present manuscript, part funded by NIHR BRC. AMZ reports personal fees from Pfizer, Janssen, Astellas, MSD and EUSA Pharma; and support for attendance of a conference from Bayer. MKBP reports, as CTU director, research funding to MRC Clinical Trials Unit from: Abcodia Pvt Ltd, Akagera, Amgen, Aspirin Foundation, Astellas, AstraZeneca, Baxter, Bayer, BMS US, Bri-BioCepheid, Cipla, Clovis Inc, CSL Behring, Eli-Lilly, Emergent Biosolutions, Gilead Sciences, GlaxoSmithKline, Grifols, Janssen Products LP, Janssen-Cilag, Johnson & Johnson, Micronoma, Modus Theraputics, Mylan, Pfizer, Sanofi, Serum Institute of India, shionogi, SyntenyBiotechnology, Takeda, Tibotec, Transgene, ViiVHealthcare, Virco and Xenothera. MRS reports personal speaker fees from Janssen, Eli Lilly and Eisai; and research grants to the institution from Astellas, Clovis Oncology, Janssen, Novartis, Pfizer and Sanofi. All other authors have declared no conflicts of interest.
Figures


References
-
- Vatne K, Stensvold A, Myklebust TA, et al. Pre- and post-prostatectomy variables associated with pelvic post-operative radiotherapy in prostate cancer patients: a national registry-based study. Acta Oncol. 2017;56(10):1295–1301. - PubMed
-
- Bolla M, van Poppel H, Collette L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911) Lancet. 2005;366(9485):572–578. - PubMed
-
- Bolla M, van Poppel H, Tombal B, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911) Lancet. 2012;380(9858):2018–2027. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

