Large conductance voltage-and calcium-activated K+ (BK) channel in health and disease
- PMID: 38584598
- PMCID: PMC10995336
- DOI: 10.3389/fphar.2024.1373507
Large conductance voltage-and calcium-activated K+ (BK) channel in health and disease
Abstract
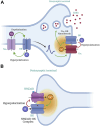
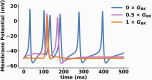
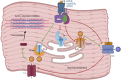
Large Conductance Voltage- and Calcium-activated K+ (BK) channels are transmembrane pore-forming proteins that regulate cell excitability and are also expressed in non-excitable cells. They play a role in regulating vascular tone, neuronal excitability, neurotransmitter release, and muscle contraction. Dysfunction of the BK channel can lead to arterial hypertension, hearing disorders, epilepsy, and ataxia. Here, we provide an overview of BK channel functioning and the implications of its abnormal functioning in various diseases. Understanding the function of BK channels is crucial for comprehending the mechanisms involved in regulating vital physiological processes, both in normal and pathological conditions, controlled by BK. This understanding may lead to the development of therapeutic interventions to address BK channelopathies.
Keywords: BK channel; KCNMA1; cellular excitability; channelopathies; molecular and cellular mechanisms; physiology.
Copyright © 2024 Echeverría, Gonzalez-Sanabria, Alvarado-Sanchez, Fernández, Castillo and Latorre.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor DT declared a past co-authorship with the author RL. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
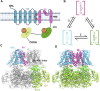
References
-
- Aaronson P. I., Sarwar U., Gin S., Rockenbauch U., Connolly M., Tillet A., et al. (2006). A role for voltage-gated, but not Ca 2+-activated, K + channels in regulating spontaneous contractile activity in myometrium from virgin and pregnant rats. Br. J. Pharmacol. 147 (7), 815–824. 10.1038/sj.bjp.0706644 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

