Identification of Keratinocyte Cytoprotectants against Toxicity by the Multikinase Inhibitor Sorafenib Using Drug Repositioning
- PMID: 38585194
- PMCID: PMC10990978
- DOI: 10.1016/j.xjidi.2024.100271
Identification of Keratinocyte Cytoprotectants against Toxicity by the Multikinase Inhibitor Sorafenib Using Drug Repositioning
Abstract
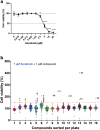
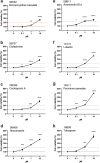
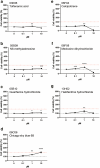
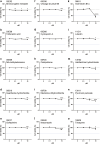
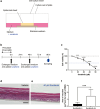
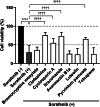
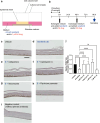
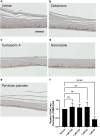
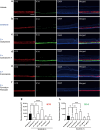
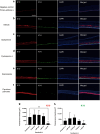
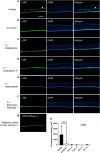
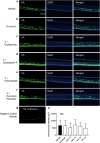
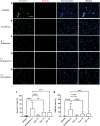
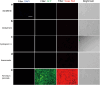
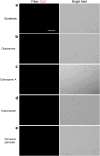
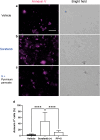
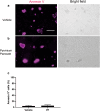
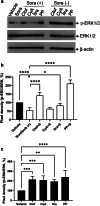
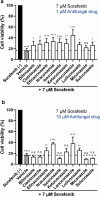
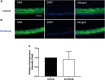
Hand-foot skin reaction is the most common adverse event of multikinase inhibitors, such as sorafenib. Although hand-foot skin reaction is not life threatening, severe cases impair quality of life because of pain and reduced activities of daily living. However, the pathological mechanisms of hand-foot skin reaction have not yet been elucidated in detail, and there is currently no effective treatment. We aimed to identify keratinocyte cytoprotectants against sorafenib toxicity. The screening of cytoprotectants against sorafenib toxicity was performed using cultured normal human epidermal keratinocytes or a reconstructed human epidermis model and off-patent approved drugs in the Prestwick Chemical library. Among 1273 drugs in the chemical library, 8 dose-dependently increased cell viability by >200% in the presence of sorafenib. In the presence of sorafenib, the number of proliferating cell nuclear antigen-positive cells was significantly higher in clofazimine-, cyclosporin A-, and itraconazole-treated reconstructed human epidermis models than in sorafenib-treated models, and candidate drugs suppressed sorafenib-induced apoptosis in normal human epidermal keratinocytes. In addition, clofazimine, itraconazole, and pyrvinium pamoate significantly recovered the phosphorylation of extracellular signal-regulated kinase 1/2 in the presence of sorafenib. Collectively, hit drugs promoted cell viability and normalized keratinocyte proliferation in the presence of sorafenib. These candidate drugs have potential as treatments for multikinase inhibitor-induced hand-foot skin reaction.
Keywords: Apoptosis; Drug development; Hand-foot skin reaction; Keratinocyte; Multikinase inhibitor.
© 2024 The Authors.
Figures


Similar articles
-
Possible Clinical Effects of Ketoconazole on Sorafenib-induced Hand-Foot Skin Reaction and Cytoprotection Mechanisms of Antifungal Agents against Multikinase Inhibitor-induced Keratinocyte Toxicity.Acta Derm Venereol. 2025 Apr 28;105:adv40697. doi: 10.2340/actadv.v105.40697. Acta Derm Venereol. 2025. PMID: 40289816 Free PMC article.
-
Hand-foot skin reaction in patients treated with sorafenib: a clinicopathological study of cutaneous manifestations due to multitargeted kinase inhibitor therapy.Br J Dermatol. 2008 Mar;158(3):592-6. doi: 10.1111/j.1365-2133.2007.08357.x. Epub 2007 Dec 6. Br J Dermatol. 2008. PMID: 18070211
-
Prostaglandin E1 reduces the keratinocyte toxicity of sorafenib by maintaining signal transducer and activator of transcription 3 (STAT3) activity and enhancing the cAMP response element binding protein (CREB) activity.Biochem Biophys Res Commun. 2017 Apr 1;485(2):227-233. doi: 10.1016/j.bbrc.2017.02.107. Epub 2017 Feb 24. Biochem Biophys Res Commun. 2017. PMID: 28237700
-
Clinical presentation and management of hand-foot skin reaction associated with sorafenib in combination with cytotoxic chemotherapy: experience in breast cancer.Oncologist. 2011;16(11):1508-19. doi: 10.1634/theoncologist.2011-0115. Epub 2011 Oct 20. Oncologist. 2011. PMID: 22016478 Free PMC article. Review.
-
Sorafenib: a review of its use in advanced hepatocellular carcinoma.Drugs. 2009;69(2):223-40. doi: 10.2165/00003495-200969020-00006. Drugs. 2009. PMID: 19228077 Review.
Cited by
-
Possible Clinical Effects of Ketoconazole on Sorafenib-induced Hand-Foot Skin Reaction and Cytoprotection Mechanisms of Antifungal Agents against Multikinase Inhibitor-induced Keratinocyte Toxicity.Acta Derm Venereol. 2025 Apr 28;105:adv40697. doi: 10.2340/actadv.v105.40697. Acta Derm Venereol. 2025. PMID: 40289816 Free PMC article.
References
-
- Adnane L., Trail P.A., Taylor I., Wilhelm S.M. Sorafenib (BAY 43-9006, Nexavar), a dual-action inhibitor that targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases VEGFR/PDGFR in tumor vasculature. Methods Enzymol. 2006;407:597–612. - PubMed
-
- Ahmadi A., Mohammadnejadi E., Karami P., Razzaghi-Asl N. Current status and structure activity relationship of privileged azoles as antifungal agents (2016–2020) Int J Antimicrob Agents. 2022;59 - PubMed
-
- Amor K.T., Ryan C., Menter A. The use of cyclosporine in dermatology: part I. J Am Acad Dermatol. 2010;63:925–946. quiz 947. - PubMed
-
- Arbiser J.L., Moschella S.L. Clofazimine: a review of its medical uses and mechanisms of action. J Am Acad Dermatol. 1995;32:241–247. - PubMed
LinkOut - more resources
Full Text Sources

