Facial palsy after administration of immune checkpoint inhibitors: case report, literature review and clinical care management
- PMID: 38585263
- PMCID: PMC10995231
- DOI: 10.3389/fimmu.2024.1375497
Facial palsy after administration of immune checkpoint inhibitors: case report, literature review and clinical care management
Abstract
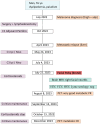
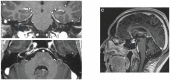
Neurological immune-related adverse events (irAEs) due to immune checkpoint inhibitors (ICI) are rare complications of immunotherapy, particularly dreadful for patients and clinical teams. Indeed, neurological irAEs are potentially severe and their diagnosis require prompt recognition and treatment. Additionally, the spectrum of neurological irAEs is broad, affecting either neuromuscular junction, peripheral or central nervous system. Here, we described the case of a 55-year man with metastatic melanoma, facing a brutal right peripheral cerebral palsy after his third ipilimumab/nivolumab infusion. After the case presentation, we reviewed the literature about this rare complication of immunotherapy, and described its diagnosis work-up and clinical management.
Keywords: cerebral palsy; immune checkpoint inhibitors; immune toxicity; ipilimumab; neurological toxicity; nivolumab.
Copyright © 2024 Mezni, Corazza, Mari, Coze, Charrier, Chanez, Chretien and Rochigneux.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Beaufils M, Amodru V, Tejeda M, Boher JM, Zemmour C, Chanez B, et al. . Dysthyroidism during immune checkpoint inhibitors is associated with improved overall survival in adult cancers: data mining of 1385 electronic patient records. J Immunother Cancer. (2023) 11:e006786. doi: 10.1136/jitc-2023-006786 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

