Biomimetic Frustrated Lewis Pair Catalysts for Hydrogenation of CO to Methanol at Low Temperatures
- PMID: 38585511
- PMCID: PMC10996047
- DOI: 10.1021/acsorginorgau.3c00064
Biomimetic Frustrated Lewis Pair Catalysts for Hydrogenation of CO to Methanol at Low Temperatures
Abstract
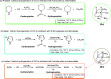
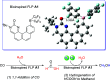
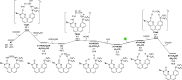
The industrial production of methanol through CO hydrogenation using the Cu/ZnO/Al2O3 catalyst requires harsh conditions, and the development of new catalysts with low operating temperatures is highly desirable. In this study, organic biomimetic FLP catalysts with good tolerance to CO poison are theoretically designed. The base-free catalytic reaction contains the 1,1-addition of CO into a formic acid intermediate and the hydrogenation of the formic acid intermediate into methanol. Low-energy spans (25.6, 22.1, and 20.6 kcal/mol) are achieved, indicating that CO can be hydrogenated into methanol at low temperatures. The new extended aromatization-dearomatization effect involving multiple rings is proposed to effectively facilitate the rate-determining CO 1,1-addition step, and a new CO activation model is proposed for organic catalysts.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






Similar articles
-
CO2 Activation and Hydrogenation on Cu-ZnO/Al2O3 Nanorod Catalysts: An In Situ FTIR Study.Nanomaterials (Basel). 2022 Jul 23;12(15):2527. doi: 10.3390/nano12152527. Nanomaterials (Basel). 2022. PMID: 35893495 Free PMC article.
-
Dual active sites over Cu-ZnO-ZrO2 catalysts for carbon dioxide hydrogenation to methanol.J Environ Sci (China). 2023 Sep;131:162-172. doi: 10.1016/j.jes.2022.10.002. Epub 2022 Oct 17. J Environ Sci (China). 2023. PMID: 37225377
-
Comparative study on the effect of different copper loading on catalytic behaviors and activity of Cu/ZnO/Al2O3 catalysts toward CO and CO2 hydrogenation.Heliyon. 2021 Jul 28;7(7):e07682. doi: 10.1016/j.heliyon.2021.e07682. eCollection 2021 Jul. Heliyon. 2021. PMID: 34386633 Free PMC article.
-
Hydrogenation of Carbon Dioxide to Methanol over Non-Cu-based Heterogeneous Catalysts.ChemSusChem. 2020 Dec 7;13(23):6160-6181. doi: 10.1002/cssc.202002054. Epub 2020 Nov 17. ChemSusChem. 2020. PMID: 33146940 Review.
-
Methanol Synthesis from CO2 Hydrogenation.ChemCatChem. 2019 Sep 5;11(17):4238-4246. doi: 10.1002/cctc.201900401. Epub 2019 Jul 10. ChemCatChem. 2019. PMID: 31894186 Free PMC article. Review.
Cited by
-
Unveiling Unusual Reactivity of SO2 and Unusual Type of S-X Long Bonds.Inorg Chem. 2025 Jul 21;64(28):14684-14692. doi: 10.1021/acs.inorgchem.5c02435. Epub 2025 Jul 8. Inorg Chem. 2025. PMID: 40626898 Free PMC article.
References
-
- Turner J. M. The matter of a clean energy future. Science 2022, 376, 1361.10.1126/science.add5094. - DOI
-
- Zhang Z.; Mao C.; Meira D. M.; Duchesne P. N.; Tountas A. A.; Li Z.; Qiu C.; Tang S.; Song R.; Ding X.; Sun J.; Yu J.; Howe J. Y.; Tu W.; Wang L.; Ozin G. A. New black indium oxide-tandem photothermal CO2-H2 methanol selective catalyst. Nat. Commun. 2022, 13, 1512.10.1038/s41467-022-29222-7. - DOI - PMC - PubMed
-
- Amann P.; Klötzer B.; Degerman D.; Köpfle N.; Götsch T.; Lömker P.; Rameshan C.; Ploner K.; Bikaljevic D.; Wang H. Y.; Soldemo M.; Shipilin M.; Goodwin C. M.; Gladh J.; Halldin Stenlid J.; Börner M.; Schlueter C.; Nilsson A. The state of zinc in methanol synthesis over a Zn/ZnO/Cu(211) model catalyst. Science 2022, 376, 603–608. 10.1126/science.abj7747. - DOI - PubMed
LinkOut - more resources
Full Text Sources
