Messenger RNA-encoded antibody approach for targeting extracellular and intracellular tau
- PMID: 38585667
- PMCID: PMC10996922
- DOI: 10.1093/braincomms/fcae100
Messenger RNA-encoded antibody approach for targeting extracellular and intracellular tau
Abstract
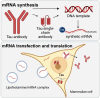
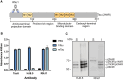
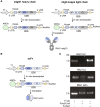
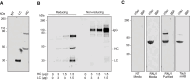
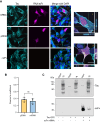
Monoclonal antibodies have emerged as a leading therapeutic agent for the treatment of disease, including Alzheimer's disease. In the last year, two anti-amyloid monoclonal antibodies, lecanemab and aducanumab, have been approved in the USA for the treatment of Alzheimer's disease, whilst several tau-targeting monoclonal antibodies are currently in clinical trials. Such antibodies, however, are expensive and timely to produce and require frequent dosing regimens to ensure disease-modifying effects. Synthetic in vitro-transcribed messenger RNA encoding antibodies for endogenous protein expression holds the potential to overcome many of the limitations associated with protein antibody production. Here, we have generated synthetic in vitro-transcribed messenger RNA encoding a tau-specific antibody as a full-sized immunoglobulin and as a single-chain variable fragment. In vitro transfection of human neuroblastoma SH-SY5Y cells demonstrated the ability of the synthetic messenger RNA to be translated into a functional tau-specific antibody. Furthermore, we show that the translation of the tau-specific single-chain variable fragment as an intrabody results in the specific engagement of intracellular tau. This work highlights the utility of messenger RNA for the delivery of antibody therapeutics, including intrabodies, for the targeting of tau in Alzheimer's disease and other tauopathies.
Keywords: Alzheimer’s disease; antibody; immunotherapy; mRNA; tau.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures

References
-
- Bajracharya R, Cruz E, Götz J, Nisbet RM. Ultrasound-mediated delivery of novel tau-specific monoclonal antibody enhances brain uptake but not therapeutic efficacy. J Control Release. 2022;349:634–648. - PubMed
-
- Ittner A, Bertz J, Suh LS, Stevens CH, Gotz J, Ittner LM. Tau-targeting passive immunization modulates aspects of pathology in tau transgenic mice. J Neurochem. 2015;132(1):135–145. - PubMed
LinkOut - more resources
Full Text Sources
