This is a preprint.
Paraneoplastic renal dysfunction in fly cancer models driven by inflammatory activation of stem cells
- PMID: 38585959
- PMCID: PMC10996499
- DOI: 10.1101/2024.03.21.586173
Paraneoplastic renal dysfunction in fly cancer models driven by inflammatory activation of stem cells
Update in
-
Paraneoplastic renal dysfunction in fly cancer models driven by inflammatory activation of stem cells.Proc Natl Acad Sci U S A. 2024 Oct 15;121(42):e2405860121. doi: 10.1073/pnas.2405860121. Epub 2024 Oct 11. Proc Natl Acad Sci U S A. 2024. PMID: 39392665 Free PMC article.
Abstract
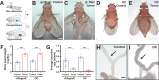
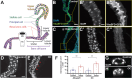
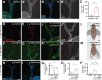
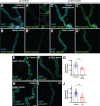
Tumors can induce systemic disturbances in distant organs, leading to physiological changes that enhance host morbidity. In Drosophila cancer models, tumors have been known for decades to cause hypervolemic 'bloating' of the abdominal cavity. Here we use allograft and transgenic tumors to show that hosts display fluid retention associated with autonomously defective secretory capacity of fly renal tubules, which function analogous to those of the human kidney. Excretion from these organs is blocked by abnormal cells that originate from inappropriate activation of normally quiescent renal stem cells (RSCs). Blockage is initiated by IL-6-like oncokines that perturb renal water-transporting cells, and trigger a damage response in RSCs that proceeds pathologically. Thus, a chronic inflammatory state produced by the tumor causes paraneoplastic fluid dysregulation by altering cellular homeostasis of host renal units.
Keywords: Biological Sciences; Drosophila; Physiology; Tumor-host; cancer model; kidney; paraneoplasia.
Figures
Similar articles
-
Paraneoplastic renal dysfunction in fly cancer models driven by inflammatory activation of stem cells.Proc Natl Acad Sci U S A. 2024 Oct 15;121(42):e2405860121. doi: 10.1073/pnas.2405860121. Epub 2024 Oct 11. Proc Natl Acad Sci U S A. 2024. PMID: 39392665 Free PMC article.
-
Mechanistic characterization of a Drosophila model of paraneoplastic nephrotic syndrome.Nat Commun. 2024 Feb 9;15(1):1241. doi: 10.1038/s41467-024-45493-8. Nat Commun. 2024. PMID: 38336808 Free PMC article.
-
Drosophila renal stem cells enhance fitness by delayed remodeling of adult Malpighian tubules.Sci Adv. 2022 May 20;8(20):eabn7436. doi: 10.1126/sciadv.abn7436. Epub 2022 May 20. Sci Adv. 2022. PMID: 35594355 Free PMC article.
-
The systemic-level repercussions of cancer-associated inflammation mediators produced in the tumor microenvironment.Front Endocrinol (Lausanne). 2022 Aug 22;13:929572. doi: 10.3389/fendo.2022.929572. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36072935 Free PMC article. Review.
-
Modeling Renal Disease "On the Fly".Biomed Res Int. 2018 May 31;2018:5697436. doi: 10.1155/2018/5697436. eCollection 2018. Biomed Res Int. 2018. PMID: 29955604 Free PMC article. Review.
References
-
- Darnell R. B., Posner J. B., Paraneoplastic Syndromes (Oxford University Press, 2010).
-
- Yeom E., et al., Tumour-derived Dilp8/INSL3 induces cancer anorexia by regulating feeding neuropeptides via Lgr3/8 in the brain. Nat Cell Biol 23, 172–183, (2021). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
