This is a preprint.
Thermotherapy has Sexually Dimorphic Responses in APP/PS1 Mice
- PMID: 38586039
- PMCID: PMC10996586
- DOI: 10.1101/2024.03.26.586836
Thermotherapy has Sexually Dimorphic Responses in APP/PS1 Mice
Update in
-
Thermotherapy has sexually dimorphic responses in APP/PS1 mice.Aging (Albany NY). 2024 Nov 29;16(21):13237-13251. doi: 10.18632/aging.206156. Epub 2024 Nov 29. Aging (Albany NY). 2024. PMID: 39614130 Free PMC article.
Abstract
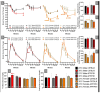
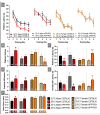
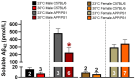
A thermoregulatory decline occurs with age due to changes in muscle mass, vasoconstriction, and metabolism that lowers core body temperature (Tc). Although lower Tc is a biomarker of successful aging, we have previously shown this worsens cognitive performance in the APP/PS1 mouse model of Alzheimer's disease (AD) [1]. We hypothesized that elevating Tc with thermotherapy would improve metabolism and cognition in APP/PS1 mice. From 6-12 months of age, male and female APP/PS1 and C57BL/6 mice were chronically housed at 23 or 30°C. At 12 months of age, mice were assayed for insulin sensitivity, glucose tolerance, and spatial cognition. Plasma, hippocampal, and peripheral (adipose, hepatic, and skeletal muscle) samples were procured postmortem and tissue-specific markers of amyloid accumulation, metabolism, and inflammation were assayed. Chronic 30°C exposure increased Tc in all groups except female APP/PS1 mice. All mice receiving thermotherapy had either improved glucose tolerance or insulin sensitivity, but the underlying processes responsible for these effects varied across sexes. In males, glucose regulation was influenced predominantly by hormonal signaling in plasma and skeletal muscle glucose transporter 4 expression, whereas in females, this was modulated at the tissue level. Thermotherapy improved spatial navigation in male C57BL/6 and APP/PS1 mice, with the later attributed to reduced hippocampal soluble amyloid-β (Aβ)42. Female APP/PS1 mice exhibited worse spatial memory recall after chronic thermotherapy. Together, the data highlights the metabolic benefits of passive thermotherapy, but future studies are needed to determine therapeutic benefits for those with AD.
Keywords: Alzheimer’s disease; cognition; core body temperature; glucose tolerance; insulin sensitivity; metabolism.
Conflict of interest statement
Conflict of Interest The authors declare no conflicts of interest.
Figures



References
-
- McFadden S, Sime LN, Cox MF, Findley CA, Peck MR, Quinn K, Fang Y, Bartke A, Hascup ER, Hascup KN. Chronic, Mild Hypothermic Environmental Temperature Does Not Ameliorate Cognitive Deficits in an Alzheimer’s Disease Mouse. Anderson RM, editor. The Journals of Gerontology: Series A [Internet]. J Gerontol A Biol Sci Med Sci; 2022. [cited 2023 Jan 24]; . Available from: 10.1093/gerona/glac223/6832816 - DOI - PMC - PubMed
-
- Basu R, Breda E, Oberg AL, Powell CC, Man CD, Basu A, Vittone JL, Klee GG, Arora P, Jensen MD, Toffolo G, Cobelli C, Rizza RA. Mechanisms of the age-associated deterioration in glucose tolerance: Contribution of alterations in insulin secretion, action, and clearance. Diabetes. American Diabetes Association Inc.; 2003; 52: 1738–48. - PubMed
-
- Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, Tobin JD, Muller D, Metter EJ. Biomarkers of caloric restriction may predict longevity in humans. Science. Science; 2002. p. 811. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
