Human immunoglobulin in combination with antimicrobial agents enhances the treatment efficacy and reduces inflammatory response in children with severe pneumonia
- PMID: 38586114
- PMCID: PMC10994785
- DOI: 10.62347/KQUW5330
Human immunoglobulin in combination with antimicrobial agents enhances the treatment efficacy and reduces inflammatory response in children with severe pneumonia
Abstract
Objective: To investigate the efficacy of human immunoglobulin combined with antibiotics in treating severe pediatric pneumonia.
Methods: A retrospective analysis was performed on 210 pediatric patients with severe pneumonia admitted to the Department of Neonatology of Cangzhou Central Hospital from April 2019 to October 2022. Patients were divided into two groups (the observation group and the control group) based on the administration of human immunoglobulin. Clinical indexes of both groups before and after treatment were analyzed to determine the therapeutic effect of different treatment methods on pediatric severe pneumonia.
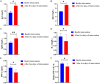
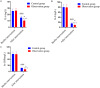
Results: The durations of cough, fever, pulmonary rales, and lung shadow, and hospitalization time in the observation group were significantly shorter than those in the control group (all P<0.05). The total clinical effective rate in the observation group was significantly higher than that in the control group (P<0.05). Levels of inflammatory factors (IL-6, IL-8 and hsCRP) were decreased in both groups after treatment (all P<0.05), and were lower in the observation group compared with the control group after treatment (all P<0.05). The serum levels of IgA, IgG and IgM after five days of intervention were obviously higher than those before intervention in the observation group (all P<0.05), but the serum levels of IL-4, INF-γ and INF-γ/IL-4 were obviously lower (all P<0.05). The total incidence of adverse reactions between two groups after intervention was not statistically different (P<0.05).
Conclusion: The combination of human immunoglobulin and antibiotics for the treatment of pediatric severe pneumonia is beneficial, because it improves efficacy, boosts the immune system, and reduces inflammation.
Keywords: Severe pneumonia; human immunoglobulin; immune function; inflammatory response.
AJTR Copyright © 2024.
Conflict of interest statement
None.
Figures
Similar articles
-
Clinical effect of reduning combined with gamma globulin treatment on symptom improvement serum levels of IL-6, 25-(OH)D and LDH in children with severe mycoplasma pneumonia.Pak J Med Sci. 2022 Mar-Apr;38(4Part-II):826-832. doi: 10.12669/pjms.38.4.5203. Pak J Med Sci. 2022. PMID: 35634590 Free PMC article.
-
Clinical Efficacy and Immune Function of Andrographolide Sulfonate in Children with Mycoplasma Pneumoniae Pneumonia.Clin Lab. 2023 Aug 1;69(8). doi: 10.7754/Clin.Lab.2022.220339. Clin Lab. 2023. PMID: 37560862
-
Efficacy of phentolamine combined with ambroxol aerosol inhalation in the treatment of pediatric severe pneumonia and its effect on serum IL-10 and CRP levels.Transl Pediatr. 2022 Jan;11(1):33-40. doi: 10.21037/tp-21-516. Transl Pediatr. 2022. PMID: 35242650 Free PMC article.
-
The Research on the Effect of Nutritional Stage-based Care Intervention in Elderly Patients with Severe Pneumonia.Altern Ther Health Med. 2024 Jul 19:AT11114. Online ahead of print. Altern Ther Health Med. 2024. PMID: 39038337
-
Effect of bronchoalveolar lavage on the clinical efficacy, inflammatory factors, and immune function in the treatment of refractory pneumonia in children.Transl Pediatr. 2021 Apr;10(4):921-928. doi: 10.21037/tp-21-89. Transl Pediatr. 2021. PMID: 34012841 Free PMC article.
Cited by
-
Case Report: From diagnosis to therapy: a lung ultrasound-driven precision strategy for neonatal atelectasis management.Front Pediatr. 2025 Jun 5;13:1584262. doi: 10.3389/fped.2025.1584262. eCollection 2025. Front Pediatr. 2025. PMID: 40538938 Free PMC article.
References
-
- de Benedictis FM, Kerem E, Chang AB, Colin AA, Zar HJ, Bush A. Complicated pneumonia in children. Lancet. 2020;396:786–798. - PubMed
-
- Bitnun A, Ford-Jones EL, Petric M, MacGregor D, Heurter H, Nelson S, Johnson G, Richardson S. Acute childhood encephalitis and mycoplasma pneumoniae. Clin Infect Dis. 2001;32:1674–1684. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
