The clinical use of remote parameter testing during cardiac implantable electronic devices implantation procedures: a single center, randomized, open-label, non-inferiority trial
- PMID: 38586175
- PMCID: PMC10995217
- DOI: 10.3389/fcvm.2024.1364940
The clinical use of remote parameter testing during cardiac implantable electronic devices implantation procedures: a single center, randomized, open-label, non-inferiority trial
Abstract
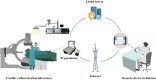
Background: A novel non-contact system for remote parameter testing and reprogramming offers an alternative method for assessing device parameters during cardiac implantable electronic devices (CIEDs) implantation without the need for physical contact with the manufacturer's clinical service technician. The safety and feasibility of using this system in CIEDs implantation procedures remains to be determined.
Objective: Evaluate the safety and feasibility of remote parameter testing in CIEDs implantation procedures.
Methods: A single center, randomized, open-label, non-inferiority trial (ChiCTR2200057587) was conducted to compare the two approaches for interrogating CIEDs during implantation procedures: routine interrogation performed by on-site technicians or remote interrogation performed by technicians using the 5G-Cloud Technology Platform. Patients aged ≥18 years and elected to receive CIEDs were eligible for inclusion. The primary endpoint was the completion rate of the parameter test. Safety and efficiency were evaluated in all randomly assigned participants.
Results: A total of 480 patients were finally enrolled and were randomly assigned to routine group (n = 240) or remote group (n = 240). The primary endpoint was achieved by 100% in both groups (P = 0.0060 for noninferiority). The parameters of sensing, threshold, and impedance regarding the right atrium, right ventricle, and left ventricle had no statistical significance between the two groups (P > 0.05). Procedure time, parameter testing time, and both duration and dose of x-ray irradiation were not significantly different between the two groups (P > 0.05). Shut-open door frequency was significantly higher in the routine group than the remote group [6.00 (4.00, 8.00) vs. 0, P < 0.0001]. Notably, no clinical or technical complications were observed in the remote group.
Conclusions: Remote parameter testing is safe and feasible across various devices implantation procedures. The utilization of remote parameter testing and reprogramming could represent an innovative approach to improve healthcare accessibility and unlock the full potential of secondary centers in managing CIEDs.
The registration identification: ChiCTR2200057587.
Keywords: COVID-19; cardiac implantable electronic devices; implantation; remote interrogation; remote reprogramming; remote testing.
© 2024 Xiong, Qin, Tong, Long, Luo, Feng, Peng, Jiang, Xiong, Li, Zhang, Zhang, Liu and Cai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Remote interrogation and reprogramming of cardiac implantable electronic devices using a custom multivendor solution.Heart Rhythm. 2023 Apr;20(4):547-551. doi: 10.1016/j.hrthm.2022.12.015. Epub 2022 Dec 13. Heart Rhythm. 2023. PMID: 36526165
-
Remote-control Interrogation, Testing, and Reprogramming of Cardiac Implantable Electronic Devices with Telehealth Protocol for the Management of Patients at Home: The DOMUM REMOTUS Study.J Innov Card Rhythm Manag. 2023 Feb 15;14(2):5348-5354. doi: 10.19102/icrm.2023.14024. eCollection 2023 Feb. J Innov Card Rhythm Manag. 2023. PMID: 36874562 Free PMC article.
-
Transvenous cardiac implantable electronic device implantation in patients with persistent left superior vena cava in a tertiary center.J Interv Card Electrophysiol. 2018 Nov;53(2):255-262. doi: 10.1007/s10840-018-0377-4. Epub 2018 May 9. J Interv Card Electrophysiol. 2018. PMID: 29740728
-
Cybersecurity in cardiac implantable electronic devices.Expert Rev Med Devices. 2019 Jun;16(6):437-444. doi: 10.1080/17434440.2019.1614440. Epub 2019 May 9. Expert Rev Med Devices. 2019. PMID: 31068028 Review.
-
Cardiac Implantable Electronic Device Implantation: Intraoperative, Acute, and Remote Complications.AACN Adv Crit Care. 2015 Oct-Dec;26(4):312-9. doi: 10.1097/NCI.0000000000000112. AACN Adv Crit Care. 2015. PMID: 26484991 Review.
References
LinkOut - more resources
Full Text Sources
Medical

