Comparative development of the serotonin- and FMRFamide-immunoreactive components of the nervous system in two distantly related ribbon worm species (Nemertea, Spiralia)
- PMID: 38586190
- PMCID: PMC10998470
- DOI: 10.3389/fnins.2024.1375208
Comparative development of the serotonin- and FMRFamide-immunoreactive components of the nervous system in two distantly related ribbon worm species (Nemertea, Spiralia)
Abstract
Introduction: Neurodevelopment in larval stages of non-model organisms, with a focus on the serotonin- and FMRFamide-immunoreactive components, has been in the focus of research in the recent past. However, some taxonomic groups remain understudied. Nemertea (ribbon worms) represent such an understudied clade with only few reports on nervous system development mostly from phylogenetically or developmentally derived species. It would be insightful to explore neurodevelopment in additional species to be able to document the diversity and deduce common patterns to trace the evolution of nervous system development.
Methods: Fluorescent immunohistochemical labeling with polyclonal primary antibodies against serotonin and FMRF-amide and a monoclonal antibody against synapsin performed on series of fixed larval stages of two nemertean species Cephalothrix rufifrons (Archinemertea, Palaeonemertea) and Emplectonema gracile (Monostilifera, Hoplonemertea) were analyzed with confocal laser scanning microscopy.
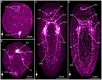
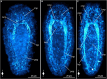
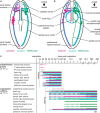
Results: This contribution gives detailed accounts on the development of the serotonin- and FMRFamide-immunoreactive subsets of the nervous system in two nemertean species from the first appearance of the respective signals. Additionally, data on synapsin-like immunoreactivity illustrates the general structure of neuropil components. Events common to both investigated species are the appearance of serotonin-like immunoreactive signals before the appearance of FMRF-like immunoreactive signals and the strict progression of the development of the lateral nerve cords from the anteriorly located, ring-shaped brain toward the posterior pole of the larva. Notable differences are (1) the proboscis nervous system that is developing much earlier in investigated larval stages of E. gracile and (2) distinct early, but apparently transient, serotonergic neurons on the frontal and caudal pole of the larva in E. gracile that seem to be absent in C. rufifrons.
Discussion: According to the results from this investigation and in line with previously published accounts on nervous system development, the hypothetical last common ancestor of Nemertea had a ring-shaped brain arranged around the proboscis opening, from which a pair of ventro-lateral nerve cords develops in anterior to posterior progression. Early frontal and caudal serotonergic neurons that later degenerate or cease to express serotonin are an ancestral character of Nemertea that they share with several other spiralian clades.
Keywords: FMRFamide; Lophotrochozoa; Nemertea; development; larva; nervous system; serotonin; synapsin.
Copyright © 2024 von Döhren.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Bartolomaeus T. (1993). “Die Leibeshöhlenverhältnisse und Verwandtschaftsbeziehungen der Spiralia (Body cavities and relationships within the Spiralia),” in Verhandlungen der Deutschen Zoologischen Gesellschaft, ed. H.-D. Pfannenstiel (Salzburg: Gustav Fischer Verlag; ), 42.
-
- Battonyai I., Voronezhskaya E. E., Obukhova A., Horváth R., Nezlin L. P., Elekes K. (2018). Neuronal development in the larvae of the invasive biofouler Dreissena polymorpha (Mollusca: Bivalvia), with special attention to sensory elements and swimming behavior. Biol. Bull. 234 192–206. 10.1086/698511 - DOI - PubMed
-
- Beckers P. (2015). The nervous systems of Pilidiophora (Nemertea). Zoomorphology 134 1–24. 10.1007/s00435-014-0246-3 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

