hsa‑miR‑455‑3P as a predictive biomarker of anemia in patients with non‑small cell lung cancer treated with carboplatin plus paclitaxel
- PMID: 38586206
- PMCID: PMC10995660
- DOI: 10.3892/ol.2024.14350
hsa‑miR‑455‑3P as a predictive biomarker of anemia in patients with non‑small cell lung cancer treated with carboplatin plus paclitaxel
Abstract
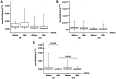
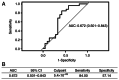
Lung cancer is the leading cause of cancer-related morbidity and mortality worldwide. The initial treatment of lung cancer depends on the definition of the tumor type and its staging. The most common treatment is chemotherapy, and the first-line treatment is a combination of carboplatin and paclitaxel. Although this treatment has good efficacy, there is a high prevalence of adverse events, particularly hematological reactions. Studies on new biomarkers related to these adverse events, such as circulating microRNAs (miRNAs/miRs), are important for optimizing the quality of life of patients. miRNAs have high stability in several biological fluids and they have specific expressions in different tissues or pathologies. Thus, the present study aimed to assess the relationship between circulating miRNAs and adverse hematologic reactions caused by treatment with carboplatin + paclitaxel in patients with lung cancer. Blood was collected from patients before and 15 days after chemotherapy for hematological adverse reaction analysis, microarray and quantitative (q)PCR validation. Adverse reactions were classified according to the Common Terminology Criteria for Adverse Events v4.0. Microarray analysis was performed using plasma from six patients without anemia and six patients with anemia, and nine miRNAs were differentially expressed. miR-1273g-3p, miR-3613-5p and miR-455-3p, identified using microarray, were assessed using qPCR in 20 patients without anemia and 26 patients with anemia. Bioinformatic analyses of miR-455-3p were performed using miRWalk, the Database for Annotation, Visualization and Integrated Discovery and GeneMania software. Microarray analysis of patients with and without anemia revealed nine significant differentially-expressed plasma miRNAs among these patients. Of these, miR-1273g-3p, miR-3613-5p and miR-455-3p were chosen for further assessment. Only miR-455-3p demonstrated a significant reduction in expression (P=0.04) between the groups before chemotherapy with carboplatin + paclitaxel. Bioinformatics analysis of miR-455-3p revealed a relationship between this miRNA and the hematopoietic pathway, particularly with respect to the RUNX family transcription factor 1 (RUNX1) and TAL bHLH transcription factor 1, erythroid differentiation factor (TAL1) genes. The most prevalent adverse reactions in patients with lung cancer treated with carboplatin + paclitaxel were hematological, particularly anemia. This adverse reaction, caused by dysfunction of the hematopoietic system, may be explained by a possible association between the important genes in this system, RUNX1 and TAL1, and hsa-miR-455-3p.
Keywords: carboplatin; hematological adverse reactions; lung cancer; microRNAs; paclitaxel.
Copyright © 2024, Spandidos Publications.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Potential biomarker of circulating hsa-miR-1273g-3p level for detection of recurrent epithelial ovarian cancer.Arch Gynecol Obstet. 2018 Dec;298(6):1173-1180. doi: 10.1007/s00404-018-4913-3. Epub 2018 Sep 27. Arch Gynecol Obstet. 2018. PMID: 30264202
-
Identification of the differential expression of genes and upstream microRNAs in small cell lung cancer compared with normal lung based on bioinformatics analysis.Medicine (Baltimore). 2020 Mar;99(11):e19086. doi: 10.1097/MD.0000000000019086. Medicine (Baltimore). 2020. PMID: 32176034 Free PMC article.
-
Clinical Significance of Circulating miR-1273g-3p and miR-122-5p in Pancreatic Cancer.Front Oncol. 2020 Feb 4;10:44. doi: 10.3389/fonc.2020.00044. eCollection 2020. Front Oncol. 2020. PMID: 32117716 Free PMC article.
-
Network pharmacology-based identification of miRNA expression of Astragalus membranaceus in the treatment of diabetic nephropathy.Medicine (Baltimore). 2022 Feb 4;101(5):e28747. doi: 10.1097/MD.0000000000028747. Medicine (Baltimore). 2022. PMID: 35119030 Free PMC article.
-
Clinical implications of miRNAs in erythropoiesis, anemia, and other hematological disorders.Mol Biol Rep. 2024 Oct 18;51(1):1064. doi: 10.1007/s11033-024-09981-w. Mol Biol Rep. 2024. PMID: 39422834 Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous
