Checkpoint Inhibitor Monotherapy in Potentially Trial-Eligible or Trial-Ineligible Patients With Metastatic NSCLC in the German Prospective CRISP Registry Real-World Cohort (AIO-TRK-0315)
- PMID: 38586301
- PMCID: PMC10995980
- DOI: 10.1016/j.jtocrr.2023.100626
Checkpoint Inhibitor Monotherapy in Potentially Trial-Eligible or Trial-Ineligible Patients With Metastatic NSCLC in the German Prospective CRISP Registry Real-World Cohort (AIO-TRK-0315)
Abstract
Introduction: Patients with metastatic NSCLC (mNSCLC) treated with immune checkpoint inhibitors in clinical practice may often not meet the strict inclusion criteria of clinical trials. Our aim was to assess the trial eligibility of patients with mNSCLC treated with pembrolizumab monotherapy in real-world and to compare the outcome of "trial-ineligible" and "potentially trial-eligible" patients.
Methods: Data from the prospective, clinical research platform CRISP were used to compare patient characteristics, treatment, and outcome of patients with programmed cell death-ligand 1 tumor proportion score greater than or equal to 50% tumors treated with pembrolizumab monotherapy who are deemed either "potentially trial-eligible" or "trial-ineligible" according to inclusion and exclusion criteria of the registrational studies (KEYNOTE-024 and -042).
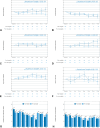
Results: Of 746 patients included, 343 patients (46.0%) were classified as "trial-ineligible" and had significantly worse outcomes compared with "potentially trial-eligible" patients (n = 403, 54.0%): median progression-free survival: 6.2 (95% confidence interval [CI]: 5.2-8.4) versus 10.3 (95% CI: 8.4-13.8) months, hazard ratio (trial-ineligible versus potentially trial-eligible) of 1.43 (95% CI: 1.19-1.72), p less than 0.001; median overall survival: 15.9 (95% CI: 11.4-20.3) versus 25.3 (95% CI: 19.8-30.4) months, hazard ratio of 1.36 (95% CI: 1.10-1.67), p equals 0.004.
Conclusions: Our data reveal that a considerable proportion of patients with mNSCLC are not eligible to participate in a clinical trial and were found to have worse outcomes than potentially trial-eligible patients, whose outcomes were comparable with those obtained from pivotal clinical trials. This is of substantial clinical relevance for physicians discussing outcomes to be expected with their patients and stresses the need for real-world effectiveness analyses.
Keywords: Immune checkpoint inhibitors; Non–small cell lung carcinoma; Pembrolizumab; Prospective studies.
© 2024 by the International Association for the Study of Lung Cancer.
Conflict of interest statement
Dr. Griesinger has received support for the present manuscript (grants to the institution) from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Takeda. Furthermore, he has received grants (to the institution) from Amgen and Siemens; personal fees for consulting from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Takeda; payment for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Takeda; support for attending meetings and/or travel from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Takeda; and personal fees for participation on a data safety monitoring board or advisory board from Merck Sharp & Dohme. He declares an unpaid fiduciary role in the German Society of Hematology and Oncology (first author of Oncopedia recommendations) and in the German Cancer Society (S3 guidelines author). Dr. Sebastian has a consulting or advisory role and has received honoraria from AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Janssen-Cilag, Eli Lilly, Merck Sharp & Dohme, Merck-Serono, Novartis, Pfizer, Roche, Takeda, and Tesaro; he has also received research funding from AstraZeneca. Dr. Brueckl has received personal fees and fees for lectures from AstraZeneca, Bristol-Myers Squibb, Boehringer, Eli Lilly, Merck Sharp & Dohme, Novartis, Pfizer, and Roche and fees for educational events from AstraZeneca, Boehringer, Eli Lilly, Pfizer, and Roche. Furthermore, he has received congress fees and personal fees from AstraZeneca, Boehringer, and Roche Pharma and has received personal fees and support for participation on advisory boards from AstraZeneca, Boehringer, Bristol-Myers Squibb, Eli Lilly Pharma, Novartis, Merck Sharp & Dohme and Roche. He declares a patent planned (EP21183549). Dr. Hummel has received personal fees as steering board member and fees for consulting from Amgen; has received personal fees for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Amgen, Bristol-Myers Squibb, and Boehringer Ingelheim; payment for expert testimony from Amgen and Boehringer Ingelheim; support for attending meetings and/or travel from Amgen and Bristol-Myers Squibb; and personal fees for participation on a data safety monitoring board or advisory board from Amgen and Boehringer Ingelheim. Dr. Kern has received support for attending meetings and/or travel from AstraZeneca, Merck Sharp & Dohme, Pfizer, and Roche and for participation on advisory boards from AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Merck/Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Takeda. Dr. Wesseler has received honoraria for lectures and travel from AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, Pfizer, Roche, Sanofi, and Takeda. Dr. Hipper and Mrs. Groth have received research funding (to their employer AIO-Studien-gGmbH) from Amgen Ltd., AstraZeneca GmbH, Boehringer Ingelheim Pharma GmbH & Co. KG, Bristol-Myers Squibb GmbH & Co. KGaA, Celgene GmbH, GlaxoSmithKline Research & Development Limited, Janssen-Cilag GmbH, Eli Lilly Deutschland GmbH, Merck Sharp & Dohme GmbH, Novartis Pharma GmbH, Pfizer Pharma GmbH, Roche Pharma AG, and Takeda Pharma Vertriebs GmbH & Co. KG. Dr. Weichert has received research support (to the institution) from AstraZeneca, Bristol-Myers Squibb, Merck Sharp & Dohme, and Roche; fees for consulting, travel support, fees for participation on advisory boards and lectures, and payment or honoraria for presentations, speakers bureaus, manuscript writing, or educational events from ADC, Agilent, Amgen, Astellas, AstraZeneca, Bayer, Boehringer, Bristol-Myers Squibb, Eisai, GlaxoSmithKline, Illumina, Janssen, Eli Lilly, Merck, Molecular Health, Merck Sharp & Dohme, Novartis, Pfizer, Siemens, Takeda, and Roche. Mr. Dörfel holds iOMEDICO shares. Dr. Schröder has received consulting fees from iOMEDICO, Celgene, Roche, Bristol-Myers Squibb, Clovis Oncology GmbH, GlaxoSmithKline, Boehringer Ingelheim, Amgen, Novartis, Merck Sharp & Dohme, AOP, Searchlight, Pharma Partner, Medixline GmbH, Eisai, HE Research GmbH, AbbVie, NIO, I+E Research, Octapharma and IPSEN. Further he declares honoraria from iOMEDICO, Celgene, Roche, Bristol-Myers Squibb, Clovis Oncology GmbH, GlaxoSmithKline, Boehringer Ingelheim, Amgen, Novartis, Merck Sharp & Dohme, AOP, Searchlight, Pharma Partner, Medixline GmbH, Eisai, HE Research GmbH, AbbVie, NIO, I+E Research, Octapharma, IPSEN, BeiGene, and Miltenyi. Dr. Eberhardt has received research funding (to the institution) from Eli Lilly, AstraZeneca, and Bristol-Myers Squibb; honoraria for advisory boards from Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Daiichi Sankyo, Janssen-Cilag, Merck/Merck Sharp & Dohme, Roche, Pfizer, Novartis, Takeda, Boehringer Ingelheim, and AbbVie; and honoraria for lectures from Amgen, Baumgart Consult, Bristol-Myers Squibb, Merck/Merck Sharp & Dohme, Roche, Pfizer, Novartis, Takeda, Boehringer Ingelheim, and AbbVie. Dr. Thomas has received research funding (to the institution) from AstraZeneca, Bristol-Myers Squibb, Merck, Roche, and Takeda; personal fees for speakers bureaus and advisory boards from Amgen, AstraZeneca, Beigene, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Chugai, Daiichi Sankyo, GlaxoSmithKline, Janssen Oncology, Eli Lilly, Merck, Merck Sharp & Dohme, Novartis, Pfizer, Roche, Sanofi, and Takeda; and support for attending meetings and/or travel from AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, Janssen Oncology, Eli Lilly, Merck, Merck Sharp & Dohme, Novartis, Pfizer, Roche, Sanofi, and Takeda. The remaining authors declare no conflict of interest.
Figures



References
-
- Planchard D., Popat S., Kerr K., et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(suppl 4):iv192–iv237. - PubMed
-
- Schiller J.H., Harrington D., Belani C.P., et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. - PubMed
LinkOut - more resources
Full Text Sources

