Maternal-fetal outcomes in patients with immune-mediated inflammatory diseases, with consideration of comorbidities: a retrospective cohort study in a large U.S. healthcare system
- PMID: 38586478
- PMCID: PMC10994966
- DOI: 10.1016/j.eclinm.2024.102435
Maternal-fetal outcomes in patients with immune-mediated inflammatory diseases, with consideration of comorbidities: a retrospective cohort study in a large U.S. healthcare system
Abstract
Background: Immune-mediated inflammatory diseases (IMIDs) are likely to complicate maternal health. However, literature on patients with IMIDs undergoing pregnancy is scarce and often overlooks the presence of comorbidities. We aimed to evaluate the impact of IMIDs on adverse pregnancy outcomes after assessing and addressing any discrepancies in the distribution of covariates associated with adverse pregnancy outcomes between patients with and without IMIDs.
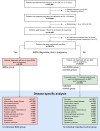
Methods: We conducted a retrospective cohort study using data from an integrated U.S. community healthcare system that provides care across Alaska, California, Montana, Oregon, New Mexico, Texas, and Washington. We used a database containing all structured data from electronic health record (EHRs) and analyzed the cohort of pregnant people who had live births from January 1, 2013, through December 31, 2022. We investigated 12 selected IMIDs: psoriasis, inflammatory bowel disease, rheumatoid arthritis, spondyloarthritis, multiple sclerosis, systemic lupus erythematosus, psoriatic arthritis, antiphospholipid syndrome, Sjögren's syndrome, vasculitides, sarcoidosis, and systemic sclerosis. We characterized patients with IMIDs prior to pregnancy (IMIDs group) based on pregnancy/maternal characteristics, comorbidities, and pre-pregnancy/prenatal immunomodulatory medications (IMMs) prescription patterns. We 1:1 propensity score matched the IMIDs cohort with people who had no IMID diagnoses prior to pregnancy (non-IMIDs cohort). Outcome measures were preterm birth (PTB), low birth weight (LBW), small for gestational age (SGA), and caesarean section.
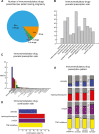
Findings: Our analytic cohort had 365,075 people, of which 5784 were in the IMIDs group and 359,291 were in the non-IMIDs group. The prevalence rate of pregnancy of at least 20 weeks duration in people with a previous IMID diagnosis has doubled in the past ten years. 17% of the IMIDs group had at least one prenatal IMM prescription. Depending on the type of IMM, 48%-70% of the patients taking IMMs before pregnancy continued them throughout pregnancy. Overall, patients with one or more of these 12 IMIDs had increased risk of PTB (Relative risk (RR) = 1.1 [1.0, 1.3]; p = 0.08), LBW (RR = 1.2 [1.0, 1.4]; p = 0.02), SGA (RR = 1.1 [1.0, 1.2]; p = 0.03), and caesarean section (RR = 1.1 [1.1, 1.2], p < 0.0001) compared to a matched cohort of people without IMIDs. When adjusted for comorbidities, patients with rheumatoid arthritis (PTB RR = 1.2, p = 0.5; LBW RR = 1.1, p = 0.6) and/or inflammatory bowel disease (PTB RR = 1.2, p = 0.3; LBW RR = 1.0, p = 0.8) did not have significantly increased risk for PTB and LBW.
Interpretation: For patients who have been pregnant for 20 weeks or greater, the association between IMIDs and adverse pregnancy outcomes depends on both the nature of the IMID and the presence of comorbidities. Because this study was limited to pregnancies resulting in live births, results must be interpreted together with other studies on early pregnancy loss and stillbirth in patient with IMIDs.
Funding: National Institutes of Health.
Keywords: Autoimmune disease; Immune mediated inflammatory disease; Immunomodulatory medications; Multiple chronic conditions; Pregnancy.
© 2024 The Author(s).
Conflict of interest statement
YH, SP, BV, QW and SM declare no conflict of interest. PM receives grant funding from Abbvie, Amgen, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, Sun Pharma, UCB. PM consults for Abbvie, Acelyrin, Aclaris, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Inmagene, Moonlake Pharama, Novartis, Sun Pharma, Union Chimique Belge. PM is the Treasurer/Secretary of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. JH has received research funding (paid to institute) from Pfizer, Novartis, Janssen, Bristol Myers Squibb and Gilead. LH is a scientific advisor for Sera Prognostics, a pregnancy diagnostics company. Sera Prognostics is not associated with this study or any of the findings.
Figures


Update of
-
Maternal-fetal outcomes in patients with immune mediated inflammatory diseases, with consideration of comorbidities: a retrospective cohort study in a large U.S. healthcare system.medRxiv [Preprint]. 2023 Aug 9:2023.08.07.23293726. doi: 10.1101/2023.08.07.23293726. medRxiv. 2023. Update in: EClinicalMedicine. 2024 Feb 01;68:102435. doi: 10.1016/j.eclinm.2024.102435. PMID: 37609126 Free PMC article. Updated. Preprint.
References
-
- Jacobson D.L., Gange S.J., Rose N.R., Graham N.M. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84(3):223–243. - PubMed
-
- Ngo S.T., Steyn F.J., McCombe P.A. Gender differences in autoimmune disease. Front Neuroendocrinol. 2014;35(3):347–369. - PubMed
-
- Betteridge J.D., Armbruster S.P., Maydonovitch C., Veerappan G.R. Inflammatory bowel disease prevalence by age, gender, race, and geographic location in the U.S. Military Health Care Population. Inflamm Bowel Dis. 2013;19(7):1421–1427. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

