Effects of RBT-1 on preconditioning response biomarkers in patients undergoing coronary artery bypass graft or heart valve surgery: a multicentre, double-blind, randomised, placebo-controlled phase 2 trial
- PMID: 38586479
- PMCID: PMC10994969
- DOI: 10.1016/j.eclinm.2023.102364
Effects of RBT-1 on preconditioning response biomarkers in patients undergoing coronary artery bypass graft or heart valve surgery: a multicentre, double-blind, randomised, placebo-controlled phase 2 trial
Abstract
Background: RBT-1 is a combination drug of stannic protoporfin (SnPP) and iron sucrose (FeS) that elicits a preconditioning response through activation of antioxidant, anti-inflammatory, and iron-scavenging pathways, as measured by heme oxygenase-1 (HO-1), interleukin-10 (IL-10), and ferritin, respectively. Our primary aim was to determine whether RBT-1 administered before surgery would safely and effectively elicit a preconditioning response in patients undergoing cardiac surgery.
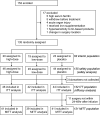
Methods: This phase 2, double-blind, randomised, placebo-controlled, parallel-group, adaptive trial, conducted in 19 centres across the USA, Canada, and Australia, enrolled patients scheduled to undergo non-emergent coronary artery bypass graft (CABG) and/or heart valve surgery with cardiopulmonary bypass. Patients were randomised (1:1:1) to receive either a single intravenous infusion of high-dose RBT-1 (90 mg SnPP/240 mg FeS), low-dose RBT-1 (45 mg SnPP/240 mg FeS), or placebo within 24-48 h before surgery. The primary outcome was a preoperative preconditioning response, measured by a composite of plasma HO-1, IL-10, and ferritin. Safety was assessed by adverse events and laboratory parameters. Prespecified adaptive criteria permitted early stopping and enrichment. This trial is registered with ClinicalTrials.gov, NCT04564833.
Findings: Between Aug 4, 2021, and Nov 9, 2022, of 135 patients who were enrolled and randomly allocated to a study group (46 high-dose, 45 low-dose, 44 placebo), 132 (98%) were included in the primary analysis (46 high-dose, 42 low-dose, 44 placebo). At interim, the trial proceeded to full enrollment without enrichment. RBT-1 led to a greater preconditioning response than did placebo at high-dose (geometric least squares mean [GLSM] ratio, 3.58; 95% CI, 2.91-4.41; p < 0.0001) and low-dose (GLSM ratio, 2.62; 95% CI, 2.11-3.24; p < 0.0001). RBT-1 was generally well tolerated by patients. The primary drug-related adverse event was dose-dependent photosensitivity, observed in 12 (26%) of 46 patients treated with high-dose RBT-1 and in six (13%) of 45 patients treated with low-dose RBT-1 (safety population).
Interpretation: RBT-1 demonstrated a statistically significant cytoprotective preconditioning response and a manageable safety profile. Further research is needed. A phase 3 trial is planned.
Funding: Renibus Therapeutics, Inc.
Keywords: Acute kidney injury; CABG; Cardiopulmonary bypass; Coronary artery bypass graft; Heart valve; Preconditioning response.
© 2023 The Author(s).
Conflict of interest statement
JBu declares consultant fees and speaker fees from 3ivelabs, Abbott, Amgen, American Regent, Applied Therapeutics, Astra Zeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardiac Dimension, Cardior, CVRx, Cytokinetics, Edwards, Element, Faraday, G3 Pharmaceutical, Imbria, Impulse Dynamics, Innolife, Inventiva, Ionis, Janssen, LivaNova, Lexicon, Medtronic, Merck, Novartis, Novo Nordisk, Otsuka, Occlutech, PharmaCosmos, Roche, Sanofi, Secretome, Sequana, Tricog, and Vifor, outside the submitted work. GMC declares consultant fees, personal fees, stock and grants as advisory board and non-profit board from Akebia, AstraZeneca, Bayer, CloudCath, CSL Behring, Durect, Eliaz Therapeutics, Mineralys, Miromatrix, NIAID, NIDDK, Outset, Physiowave, ReCor, Renibus, Sanifit, Satellite Healthcare, Unicycive, and Vertex, outside the submitted work. JBe declares personal fees and stock as advisory board and speaker fee from Anteris Pty Ltd, Medtronic, Edwards LifeSicences, Abbott Medical, Anteris, LivaNova, Johnson and Johnson, Prostheses List Advisory Committee—Dept of Health (Govt of Australia), Australia & New Zealand Society Cardiac & Thoracic Surgeons (ANZSCTS), and Cardiac Society Australia and New Zealand (CSANZ), outside the submitted work. PJD declares grants, personal fees and equipment as advisory board and consultant from Abbott Diagnostics, Roche Diagnostics, Siemens, Trimedic Canada, Renibus, PEPPER, Bayer AG, Quidel, CloudDX, and Philips Healthcare, outside the submitted work. CAM declares payment for expert testimony and is chair of the membership committee for the New York Society for Thoracic Surgery, outside the submitted work. DHS declares personal fees from Renibus Therapeutics for the submitted manuscript. BS declares personal fees from Renibus Therapeutics for the submitted manuscript and personal fees, patents, and stock as advisory board from Allakos, Nephcentric, Transplant First Academy, and Cardiorenal Society. SS declares consultant fees from Renibus for the submitted work and consultant fees and advisory board from Advarra, Allogene, Apellis, Athira, Blue Note, Equillium, Genescence, GSK, Latigo, Mironid, Moderna, Neumora, Nurix, Oculis, Prolaio, Prolocor, Sail Bio, Transcenta, University of Vermont, Acadia, Oxthera, Vertex, Wildwood, Labcorp/Fortrea, IQVIA, Syneos, PPD, ICON, Parexel, Medpace, Castle Creek, Acelyrin, Aerovate, Affinivax, Alexion, Kyverna, Moderna, Pharvaris, and Horizon, outside the submitted work. CW declares consultant fees from Renibus Therapeutics for the submitted work. RZ declares support from Renibus Therapeutics for the submitted work and grants, speaking fees, stock, and equipment from Renibus Therapeutics, outside the submitted work. SR declares support from Renibus Therapeutics for the submitted work as well as patents and stock from Renibus Therapeutics, outside the submitted work. JR declares support from Renibus Therapeutics for the submitted work and stock from Renibus Therapeutics, outside the submitted work. RZ, JR, SR, and BS are employees of Renibus Therapeutics Inc. All other authors declare no competing interests.
Figures
References
-
- Pahwa S., Bernabei A., Schaff H., et al. Impact of postoperative complications after cardiac surgery on long-term survival. J Card Surg. 2021;36(6):2045–2052. - PubMed
-
- Kim K.M., Arghami A., Habib R., et al. The society of thoracic Surgeons adult cardiac surgery database: 2022 update on outcomes and research. Ann Thorac Surg. 2023;115(3):566–574. - PubMed
-
- Hall R.I., Smith M.S., Rocker G. The systemic inflammatory response to cardiopulmonary bypass: pathophysiological, therapeutic, and pharmacological considerations. Anesth Analg. 1997;85(4):766–782. - PubMed
-
- Algoet M., Janssens S., Himmelreich U., et al. Myocardial ischemia-reperfusion injury and the influence of inflammation. Trends Cardiovasc Med. 2022;33(6):357–366. - PubMed
-
- Zakkar M., Ascione R., James A., Angelini G., Suleiman M. Inflammation, oxidative stress and postoperative atrial fibrillation in cardiac surgery. Pharmacol Ther. 2015;154:13–20. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous