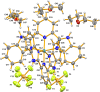
Bis[2,6-bis-(1 H-benzimidazol-2-yl)pyridine]ruthenium(II) bis(hexa-fluorido-phosphate) diethyl ether tris-olvate
- PMID: 38586523
- PMCID: PMC10993555
- DOI: 10.1107/S2414314624002694
Bis[2,6-bis-(1 H-benzimidazol-2-yl)pyridine]ruthenium(II) bis(hexa-fluorido-phosphate) diethyl ether tris-olvate
Abstract
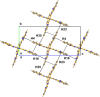
The title compound, [Ru(C19H13N5)2](PF6)2·3C4H10O, was obtained from the reaction of Ru(bimpy)Cl3 [bimpy is 2,6-bis-(1H-benzimidazol-2-yl)pyridine] and bimpy in refluxing ethanol followed by recrystallization from diethyl ether/aceto-nitrile. At 125 K the complex has ortho-rhom-bic (Pca21) symmetry. It is remarkable that the structure is almost centrosymmetric. However, refinement in space group Pbcn leads to disorder and definitely worse results. It is of inter-est with respect to potential catalytic reduction of CO2. The structure displays N-H⋯O, N-H⋯F hydrogen bonding and significant π-π stacking and C-H⋯π stacking inter-actions.
Keywords: Bimpy ligand; crystal structure; ruthenium(II) complex.
© Althubyani et al. 2024.
Figures
References
-
- Bruker (2009). APEX2 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Chen, Z., Chen, C., Weinberg, D. R., Kang, P., Concepcion, J. J., Harrison, D. P., Brookhart, M. S. & Meyer, T. J. (2011). Chem. Commun. 47, 12607–12609. - PubMed
-
- Juris, A., Balzani, V., Barigelletti, F., Campagna, S., Belser, P. & von Zelewsky, A. (1988). Coord. Chem. Rev. 84, 85–277.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous