Activation of Heme Oxygenase-1 by Mangiferin in Human Retinal Pigment Epithelial Cells Contributes to Blocking Oxidative Damage
- PMID: 38586992
- PMCID: PMC11063488
- DOI: 10.4062/biomolther.2023.175
Activation of Heme Oxygenase-1 by Mangiferin in Human Retinal Pigment Epithelial Cells Contributes to Blocking Oxidative Damage
Abstract
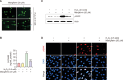
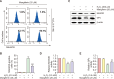
Mangiferin is a kind of natural xanthone glycosides and is known to have various pharmacological activities. However, since the beneficial efficacy of this compound has not been reported in retinal pigment epithelial (RPE) cells, this study aimed to evaluate whether mangiferin could protect human RPE ARPE-19 cells from oxidative injury mimicked by hydrogen peroxide (H2O2). The results showed that mangiferin attenuated H2O2-induced cell viability reduction and DNA damage, while inhibiting reactive oxygen species (ROS) production and preserving diminished glutathione (GSH). Mangiferin also antagonized H2O2-induced inhibition of the expression and activity of antioxidant enzymes such as manganese superoxide dismutase and GSH peroxidase, which was associated with inhibition of mitochondrial ROS production. In addition, mangiferin protected ARPE-19 cells from H2O2-induced apoptosis by increasing the Bcl-2/Bax ratio, decreasing caspase-3 activation, and blocking poly(ADP-ribose) polymerase cleavage. Moreover, mangiferin suppressed the release of cytochrome c into the cytosol, which was achieved by interfering with mitochondrial membrane disruption. Furthermore, mangiferin increased the expression and activity of heme oxygenase-1 (HO-1) and nuclear factor-erythroid-2 related factor 2 (Nrf2). However, the inhibition of ROS production, cytoprotective and anti-apoptotic effects of mangiferin were significantly attenuated by the HO-1 inhibitor, indicating that mangiferin promoted Nrf2-mediated HO-1 activity to prevent ARPE-19 cells from oxidative injury. The results of this study suggest that mangiferin, as an Nrf2 activator, has potent ROS scavenging activity and may have the potential to protect oxidative stress-mediated ocular diseases.
Keywords: Apoptosis; DNA damage; Mangiferin; Nrf2/HO-1; Reactive oxygen species.
Conflict of interest statement
The authors have no conflicts of interest relevant to this study to disclose.
Figures
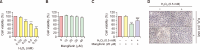






References
-
- Alberdi E., Sánchez-Gómez M. V., Ruiz A., Cavaliere F., Ortiz-Sanz C., Quintela-López T., Capetillo-Zarate E., Solé-Domènech S., Matute C. Mangiferin and morin attenuate oxidative stress, mitochondrial dysfunction, and neurocytotoxicity, induced by amyloid b oligomers. Oxid. Med. Cell. Longev. 2018;2018:2856063. doi: 10.1155/2018/2856063. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

