Alkali injury-induced pathological lymphangiogenesis in the iris facilitates the infiltration of T cells and ocular inflammation
- PMID: 38587075
- PMCID: PMC11128208
- DOI: 10.1172/jci.insight.175479
Alkali injury-induced pathological lymphangiogenesis in the iris facilitates the infiltration of T cells and ocular inflammation
Abstract
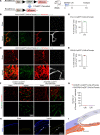
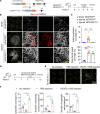
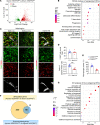
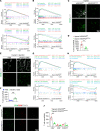
Inflammatory lymphangiogenesis is intimately linked to immune regulation and tissue homeostasis. However, current evidence has suggested that classic lymphatic vessels are physiologically absent in intraocular structures. Here, we show that neolymphatic vessels were induced in the iris after corneal alkali injury (CAI) in a VEGFR3-dependent manner. Cre-loxP-based lineage tracing revealed that these lymphatic endothelial cells (LECs) originate from existing Prox1+ lymphatic vessels. Notably, the ablation of iridial lymphangiogenesis via conditional deletion of VEGFR3 alleviated the ocular inflammatory response and pathological T cell infiltration. Our findings demonstrate that iridial neolymphatics actively participate in pathological immune responses following injury and suggest intraocular lymphangiogenesis as a valuable therapeutic target for the treatment of ocular inflammation.
Keywords: Immunology; Lymph; Ophthalmology; Retinopathy; T cells.
Figures


Similar articles
-
MicroRNA miR-466 inhibits Lymphangiogenesis by targeting prospero-related homeobox 1 in the alkali burn corneal injury model.J Biomed Sci. 2015 Jan 2;22(1):3. doi: 10.1186/s12929-014-0104-0. J Biomed Sci. 2015. PMID: 25573115 Free PMC article.
-
Simultaneous in vivo imaging of blood and lymphatic vessel growth in Prox1-GFP/Flk1::myr-mCherry mice.FEBS J. 2015 Apr;282(8):1458-1467. doi: 10.1111/febs.13234. Epub 2015 Mar 6. FEBS J. 2015. PMID: 25688651 Free PMC article.
-
Inhibition of Lymphangiogenesis and Hemangiogenesis in Corneal Inflammation by Subconjunctival Prox1 siRNA Injection in Rats.Invest Ophthalmol Vis Sci. 2015 Sep;56(10):5871-9. doi: 10.1167/iovs.14-14433. Invest Ophthalmol Vis Sci. 2015. PMID: 26348636
-
Two Birds, One Stone: Double Hits on Tumor Growth and Lymphangiogenesis by Targeting Vascular Endothelial Growth Factor Receptor 3.Cells. 2019 Mar 21;8(3):270. doi: 10.3390/cells8030270. Cells. 2019. PMID: 30901976 Free PMC article. Review.
-
The novel role of lymphatic vessels in the pathogenesis of ocular diseases.Prog Retin Eye Res. 2023 Sep;96:101157. doi: 10.1016/j.preteyeres.2022.101157. Epub 2023 Feb 8. Prog Retin Eye Res. 2023. PMID: 36759312 Review.
Cited by
-
Ferroptosis: a novel mechanism of cell death in ophthalmic conditions.Front Immunol. 2024 Jun 27;15:1440309. doi: 10.3389/fimmu.2024.1440309. eCollection 2024. Front Immunol. 2024. PMID: 38994366 Free PMC article. Review.
-
From mechanisms to medicine: Ferroptosis as a Therapeutic target in liver disorders.Cell Commun Signal. 2025 Mar 7;23(1):125. doi: 10.1186/s12964-025-02121-2. Cell Commun Signal. 2025. PMID: 40055721 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

