Neisseria gonorrhoeae scavenges host sialic acid for Siglec-mediated, complement-independent suppression of neutrophil activation
- PMID: 38587424
- PMCID: PMC11078009
- DOI: 10.1128/mbio.00119-24
Neisseria gonorrhoeae scavenges host sialic acid for Siglec-mediated, complement-independent suppression of neutrophil activation
Abstract
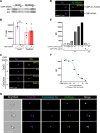
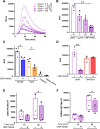
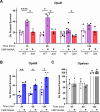
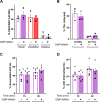
Gonorrhea, caused by the bacterium Neisseria gonorrhoeae (Gc), is characterized by neutrophilic influx to infection sites. Gc has developed mechanisms to resist killing by neutrophils that include modifications to its surface lipooligosaccharide (LOS). One such LOS modification is sialylation: Gc sialylates its terminal LOS sugars with cytidine-5'-monophosphate-N-acetylneuraminic acid, which is scavenged from the host using LOS sialyltransferase (Lst) since Gc cannot make its sialic acid. Sialylation enables sensitive strains of Gc to resist complement-mediated killing in a serum-dependent manner. However, little is known about the contribution of sialylation to complement-independent, direct Gc-neutrophil interactions. In the absence of complement, we found sialylated Gc expressing opacity-associated (Opa) proteins decreased the oxidative burst and granule exocytosis from primary human neutrophils. In addition, sialylated Opa+ Gc survived better than vehicle treated or Δlst Gc when challenged with neutrophils. However, Gc sialylation did not significantly affect Opa-dependent association with or internalization of Gc by neutrophils. Previous studies have implicated sialic acid-binding immunoglobulin-type lectins (Siglecs) in modulating neutrophil interactions with sialylated Gc. Blocking neutrophil Siglecs with antibodies that bind to their extracellular domains eliminated the ability of sialylated Opa+ Gc to suppress the oxidative burst and resist neutrophil killing. These findings highlight a new role for sialylation in Gc evasion of human innate immunity, with implications for the development of vaccines and therapeutics for gonorrhea.
Importance: Neisseria gonorrhoeae, the bacterium that causes gonorrhea, is an urgent global health concern due to increasing infection rates, widespread antibiotic resistance, and its ability to thwart protective immune responses. The mechanisms by which Gc subverts protective immune responses remain poorly characterized. One way N. gonorrhoeae evades human immunity is by adding sialic acid that is scavenged from the host onto its lipooligosaccharide, using the sialyltransferase Lst. Here, we found that sialylation enhances N. gonorrhoeae survival from neutrophil assault and inhibits neutrophil activation, independently of the complement system. Our results implicate bacterial binding of sialic acid-binding lectins (Siglecs) on the neutrophil surface, which dampens neutrophil antimicrobial responses. This work identifies a new role for sialylation in protecting N. gonorrhoeae from cellular innate immunity, which can be targeted to enhance the human immune response in gonorrhea.
Keywords: Neisseria gonorrhoeae; degranulation; gonorrhea; infection; lipooligosaccharide; neutrophils; reactive oxygen species; sialylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Update of
-
Neisseria gonorrhoeae scavenges host sialic acid for Siglec-mediated, complement-independent suppression of neutrophil activation.bioRxiv [Preprint]. 2024 Jan 18:2024.01.17.576097. doi: 10.1101/2024.01.17.576097. bioRxiv. 2024. Update in: mBio. 2024 May 8;15(5):e0011924. doi: 10.1128/mbio.00119-24. PMID: 38293026 Free PMC article. Updated. Preprint.
Similar articles
-
Neisseria gonorrhoeae scavenges host sialic acid for Siglec-mediated, complement-independent suppression of neutrophil activation.bioRxiv [Preprint]. 2024 Jan 18:2024.01.17.576097. doi: 10.1101/2024.01.17.576097. bioRxiv. 2024. Update in: mBio. 2024 May 8;15(5):e0011924. doi: 10.1128/mbio.00119-24. PMID: 38293026 Free PMC article. Updated. Preprint.
-
α-2,3-sialyltransferase expression level impacts the kinetics of lipooligosaccharide sialylation, complement resistance, and the ability of Neisseria gonorrhoeae to colonize the murine genital tract.mBio. 2015 Feb 3;6(1):e02465-14. doi: 10.1128/mBio.02465-14. mBio. 2015. PMID: 25650401 Free PMC article.
-
The Lst Sialyltransferase of Neisseria gonorrhoeae Can Transfer Keto-Deoxyoctanoate as the Terminal Sugar of Lipooligosaccharide: a Glyco-Achilles Heel That Provides a New Strategy for Vaccines to Prevent Gonorrhea.mBio. 2021 Mar 23;12(2):e03666-20. doi: 10.1128/mBio.03666-20. mBio. 2021. PMID: 33758087 Free PMC article.
-
Gonococcal Defenses against Antimicrobial Activities of Neutrophils.Trends Microbiol. 2018 Dec;26(12):1022-1034. doi: 10.1016/j.tim.2018.07.003. Epub 2018 Aug 13. Trends Microbiol. 2018. PMID: 30115561 Free PMC article. Review.
-
Pathogenesis of Neisseria gonorrhoeae in the female reproductive tract: neutrophilic host response, sustained infection, and clinical sequelae.Curr Opin Hematol. 2018 Jan;25(1):13-21. doi: 10.1097/MOH.0000000000000394. Curr Opin Hematol. 2018. PMID: 29016383 Free PMC article. Review.
Cited by
-
Chemical tools to study and modulate glycan-mediated host-bacteria interactions.Curr Opin Chem Biol. 2025 Aug;87:102603. doi: 10.1016/j.cbpa.2025.102603. Epub 2025 Jun 4. Curr Opin Chem Biol. 2025. PMID: 40472591 Review.
-
Interferon-epsilon, an estrogen-induced type I interferon, is uniquely exploited by Neisseria gonorrhoeae via effects on sialic acid metabolism.Cell Host Microbe. 2025 Jul 9;33(7):1133-1145.e4. doi: 10.1016/j.chom.2025.05.015. Epub 2025 Jun 9. Cell Host Microbe. 2025. PMID: 40494348
References
-
- World Health Organization . 2024. Gonorrhoea (Neisseria gonorrhoeae infection). Available from: https://www.who.int/news-room/fact-sheets/detail/gonorrhoea-(neisseria-g...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

