Inhibition of influenza A virus and SARS-CoV-2 infection or co-infection by griffithsin and griffithsin-based bivalent entry inhibitor
- PMID: 38587427
- PMCID: PMC11077956
- DOI: 10.1128/mbio.00741-24
Inhibition of influenza A virus and SARS-CoV-2 infection or co-infection by griffithsin and griffithsin-based bivalent entry inhibitor
Abstract
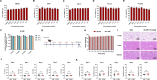
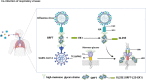
Outbreaks of acute respiratory viral diseases, such as influenza and COVID-19 caused by influenza A virus (IAV) and SARS-CoV-2, pose a serious threat to global public health, economic security, and social stability. This calls for the development of broad-spectrum antivirals to prevent or treat infection or co-infection of IAV and SARS-CoV-2. Hemagglutinin (HA) on IAV and spike (S) protein on SARS-CoV-2, which contain various types of glycans, play crucial roles in mediating viral entry into host cells. Therefore, they are key targets for the development of carbohydrate-binding protein-based antivirals. This study demonstrated that griffithsin (GRFT) and the GRFT-based bivalent entry inhibitor GL25E (GRFT-L25-EK1) showed broad-spectrum antiviral effects against IAV infection in vitro by binding to HA in a carbohydrate-dependent manner and effectively protected mice from lethal IAV infection. Although both GRFT and GL25E could inhibit infection of SARS-CoV-2 Omicron variants, GL25E proved to be significantly more effective than GRFT and EK1 alone. Furthermore, GL25E effectively inhibited in vitro co-infection of IAV and SARS-CoV-2 and demonstrated good druggability, including favorable safety and stability profiles. These findings suggest that GL25E is a promising candidate for further development as a broad-spectrum antiviral drug for the prevention and treatment of infection or co-infection from IAV and SARS-CoV-2.IMPORTANCEInfluenza and COVID-19 are highly contagious respiratory illnesses caused by the influenza A virus (IAV) and SARS-CoV-2, respectively. IAV and SARS-CoV-2 co-infection exacerbates damage to lung tissue and leads to more severe clinical symptoms, thus calling for the development of broad-spectrum antivirals for combating IAV and SARS-CoV-2 infection or co-infection. Here we found that griffithsin (GRFT), a carbohydrate-binding protein, and GL25E, a recombinant protein consisting of GRFT, a 25 amino acid linker, and EK1, a broad-spectrum coronavirus inhibitor, could effectively inhibit IAV and SARS-CoV-2 infection and co-infection by targeting glycans on HA of IAV and spike (S) protein of SARS-CoV-2. GL25E is more effective than GRFT because GL25E can also interact with the HR1 domain in SARS-CoV-2 S protein. Furthermore, GL25E possesses favorable safety and stability profiles, suggesting that it is a promising candidate for development as a drug to prevent and treat IAV and SARS-CoV-2 infection or co-infection.
Keywords: SARS-CoV-2; co-infection; griffithsin; influenza A virus; respiratory viruses.
Conflict of interest statement
L.L., S.J., N.C., X.W., and Y.C. are the inventors in the patent or patent application covering the recombinant proteins GRFT and GL25E. Other authors declare no conflict of interest.
Figures
Similar articles
-
A bivalent protein targeting glycans and HR1 domain in spike protein potently inhibited infection of SARS-CoV-2 and other human coronaviruses.Cell Biosci. 2021 Jul 8;11(1):128. doi: 10.1186/s13578-021-00638-w. Cell Biosci. 2021. PMID: 34238357 Free PMC article.
-
A small molecule compound targeting hemagglutinin inhibits influenza A virus and exhibits broad-spectrum antiviral activity.Acta Pharmacol Sin. 2024 Nov;45(11):2380-2393. doi: 10.1038/s41401-024-01331-7. Epub 2024 Jul 10. Acta Pharmacol Sin. 2024. PMID: 38987389
-
Biocompatible Iron Oxide Nanoparticles Display Antiviral Activity Against Two Different Respiratory Viruses in Mice.Int J Nanomedicine. 2024 Dec 21;19:13763-13788. doi: 10.2147/IJN.S475323. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39723174 Free PMC article.
-
Influenza A virus entry inhibitors targeting the hemagglutinin.Viruses. 2013 Jan 22;5(1):352-73. doi: 10.3390/v5010352. Viruses. 2013. PMID: 23340380 Free PMC article. Review.
-
Griffithsin: An Antiviral Lectin with Outstanding Therapeutic Potential.Viruses. 2016 Oct 24;8(10):296. doi: 10.3390/v8100296. Viruses. 2016. PMID: 27783038 Free PMC article. Review.
Cited by
-
Pseudotyped Viruses: A Useful Platform for Pre-Clinical Studies Conducted in a BSL-2 Laboratory Setting.Biomolecules. 2025 Jan 15;15(1):135. doi: 10.3390/biom15010135. Biomolecules. 2025. PMID: 39858529 Free PMC article. Review.
-
Biosimilars Targeting Pathogens: A Comprehensive Review of Their Role in Bacterial, Fungal, Parasitic, and Viral Infections.Pharmaceutics. 2025 Apr 28;17(5):581. doi: 10.3390/pharmaceutics17050581. Pharmaceutics. 2025. PMID: 40430873 Free PMC article. Review.
References
-
- Kandeil A, Patton C, Jones JC, Jeevan T, Harrington WN, Trifkovic S, Seiler JP, Fabrizio T, Woodard K, Turner JC, Crumpton JC, Miller L, Rubrum A, DeBeauchamp J, Russell CJ, Govorkova EA, Vogel P, Kim-Torchetti M, Berhane Y, Stallknecht D, Poulson R, Kercher L, Webby RJ. 2023. Rapid evolution of A(H5N1) influenza viruses after intercontinental spread to North America. Nat Commun 14:3082. doi:10.1038/s41467-023-38415-7 - DOI - PMC - PubMed
-
- Cronk BD, Caserta LC, Laverack M, Gerdes RS, Hynes K, Hopf CR, Fadden MA, Nakagun S, Schuler KL, Buckles EL, Lejeune M, Diel DG. 2023. Infection and tissue distribution of highly pathogenic avian influenza A type H5N1 (clade 2.3.4.4b) in red Fox kits (Vulpes vulpes). Emerg Microbes Infect 12:2249554. doi:10.1080/22221751.2023.2249554 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 92169112/MOST | National Natural Science Foundation of China (NSFC)
- 82341036/MOST | National Natural Science Foundation of China (NSFC)
- 2022YFC2604102/MOST | National Key Research and Development Program of China (NKPs)
- 2021YFC2300703/MOST | National Key Research and Development Program of China (NKPs)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

