Attitudes Toward COVID-19 Vaccines Among Pregnant and Recently Pregnant Individuals
- PMID: 38587844
- PMCID: PMC11002697
- DOI: 10.1001/jamanetworkopen.2024.5479
Attitudes Toward COVID-19 Vaccines Among Pregnant and Recently Pregnant Individuals
Abstract
Importance: Pregnant people and infants are at high risk of severe COVID-19 outcomes. Understanding changes in attitudes toward COVID-19 vaccines among pregnant and recently pregnant people is important for public health messaging.
Objective: To assess attitudinal trends regarding COVID-19 vaccines by (1) vaccination status and (2) race, ethnicity, and language among samples of pregnant and recently pregnant Vaccine Safety Datalink (VSD) members from 2021 to 2023.
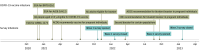
Design, setting, and participants: This cross-sectional surveye study included pregnant or recently pregnant members of the VSD, a collaboration of 13 health care systems and the US Centers for Disease Control and Prevention. Unvaccinated, non-Hispanic Black, and Spanish-speaking members were oversampled. Wave 1 took place from October 2021 to February 2022, and wave 2 took place from November 2022 to February 2023. Data were analyzed from May 2022 to September 2023.
Exposures: Self-reported or electronic health record (EHR)-derived race, ethnicity, and preferred language.
Main outcomes and measures: Self-reported vaccination status and attitudes toward monovalent (wave 1) or bivalent Omicron booster (wave 2) COVID-19 vaccines. Sample- and response-weighted analyses assessed attitudes by vaccination status and 3 race, ethnicity, and language groupings of interest.
Results: There were 1227 respondents; all identified as female, the mean (SD) age was 31.7 (5.6) years, 356 (29.0%) identified as Black race, 555 (45.2%) identified as Hispanic ethnicity, and 445 (36.3%) preferred the Spanish language. Response rates were 43.5% for wave 1 (652 of 1500 individuals sampled) and 39.5% for wave 2 (575 of 1456 individuals sampled). Respondents were more likely than nonrespondents to be White, non-Hispanic, and vaccinated per EHR. Overall, 76.8% (95% CI, 71.5%-82.2%) reported 1 or more COVID-19 vaccinations; Spanish-speaking Hispanic respondents had the highest weighted proportion of respondents with 1 or more vaccination. Weighted estimates of somewhat or strongly agreeing that COVID-19 vaccines are safe decreased from wave 1 to 2 for respondents who reported 1 or more vaccinations (76% vs 50%; χ21 = 7.8; P < .001), non-Hispanic White respondents (72% vs 43%; χ21 = 5.4; P = .02), and Spanish-speaking Hispanic respondents (76% vs 53%; χ21 = 22.8; P = .002).
Conclusions and relevance: Decreasing confidence in COVID-19 vaccine safety in a large, diverse pregnant and recently pregnant insured population is a public health concern.
Conflict of interest statement
Figures
References
-
- Zambrano LD, Ellington S, Strid P, et al. ; CDC COVID-19 Response Pregnancy and Infant Linked Outcomes Team . Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1641-1647. doi:10.15585/mmwr.mm6944e3 - DOI - PMC - PubMed
-
- Allotey J, Stallings E, Bonet M, et al. ; for PregCOV-19 Living Systematic Review Consortium . Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi:10.1136/bmj.m3320 - DOI - PMC - PubMed
-
- Galang RR, Newton SM, Woodworth KR, et al. ; Centers for Disease Control and Prevention COVID-19 Response Pregnancy and Infant Linked Outcomes Team . Risk factors for illness severity among pregnant women with confirmed severe acute respiratory syndrome coronavirus 2 infection-surveillance for emerging threats to mothers and babies network, 22 state, local, and territorial health departments, 29 March 2020-5 March 2021. Clin Infect Dis. 2021;73(suppl 1):S17-S23. doi:10.1093/cid/ciab432 - DOI - PMC - PubMed
-
- Woodworth KR, Olsen EO, Neelam V, et al. ; CDC COVID-19 Response Pregnancy and Infant Linked Outcomes Team; COVID-19 Pregnancy and Infant Linked Outcomes Team (PILOT) . Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy—SET-NET, 16 jurisdictions, March 29-October 14, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1635-1640. doi:10.15585/mmwr.mm6944e2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

