Pregnancy is linked to faster epigenetic aging in young women
- PMID: 38588424
- PMCID: PMC11032455
- DOI: 10.1073/pnas.2317290121
Pregnancy is linked to faster epigenetic aging in young women
Abstract
A central prediction of evolutionary theory is that energy invested into reproduction comes at the expense of somatic maintenance and repair, accelerating biological aging. Supporting this prediction are findings that high fertility among women predicts shorter lifespan and poorer health later in life. However, biological aging is thought to begin before age-related health declines, limiting the applicability of morbidity and mortality for studying the aging process earlier in life. Here, we examine the relationship between reproductive history and biological aging in a sample of young (20 to 22yo) men and women from the Cebu Longitudinal Health and Nutrition Survey, located in the Philippines (n = 1,735). We quantify biological aging using six measures, collectively known as epigenetic clocks, reflecting various facets of cellular aging, health, and mortality risk. In a subset of women, we test whether longitudinal changes in gravidity between young and early-middle adulthood (25 to 31yo) are associated with changes in epigenetic aging during that time. Cross-sectionally, gravidity was associated with all six measures of accelerated epigenetic aging in women (n = 825). Furthermore, longitudinal increases in gravidity were linked to accelerated epigenetic aging in two epigenetic clocks (n = 331). In contrast, the number of pregnancies a man reported fathering was not associated with epigenetic aging among same-aged cohort men (n = 910). These effects were robust to socioecological, environmental, and immunological factors, consistent with the hypothesis that pregnancy accelerates biological aging and that these effects can be detected in young women in a high-fertility context.
Keywords: biological aging; costs of reproduction; fatherhood; pregnancy.
Conflict of interest statement
Competing interests statement:D.W.B. is listed as an inventor on a Duke University and University of Otago invention, DunedinPACE, that was licensed to a commercial entity.
Figures
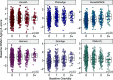
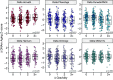
Comment in
-
A life for a (shorter) life: The reproduction-longevity trade-off.Proc Natl Acad Sci U S A. 2024 Apr 23;121(17):e2405089121. doi: 10.1073/pnas.2405089121. Epub 2024 Apr 10. Proc Natl Acad Sci U S A. 2024. PMID: 38598350 Free PMC article. No abstract available.
References
-
- Williams G. C., Natural Selection, the Costs of Reproduction, and a Refinement of Lack’s Principle. Am. Nat. 100, 687–690 (1966).
-
- Kirkwood T. B. L., Evolution of ageing. Nature. 270, 301–304 (1977). - PubMed
-
- Dijkstra C., et al. , Brood Size Manipulations in the Kestrel (Falco tinnunculus): Effects on Offspring and Parent Survival. J. Anim. Ecol. 59, 269–285 (1990).
-
- Obeso J. R., The costs of reproduction in plants. New Phytol. 155, 321–348 (2002). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

