Randomized Placebo-Controlled, Biomarker-Stratified Phase Ib Microbiome Modulation in Melanoma: Impact of Antibiotic Preconditioning on Microbiome and Immunity
- PMID: 38588588
- PMCID: PMC11215408
- DOI: 10.1158/2159-8290.CD-24-0066
Randomized Placebo-Controlled, Biomarker-Stratified Phase Ib Microbiome Modulation in Melanoma: Impact of Antibiotic Preconditioning on Microbiome and Immunity
Abstract
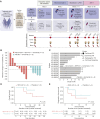
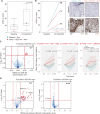
Gut-microbiota modulation shows promise in improving immune-checkpoint blockade (ICB) response; however, precision biomarker-driven, placebo-controlled trials are lacking. We performed a multicenter, randomized placebo-controlled, biomarker-stratified phase I trial in patients with ICB-naïve metastatic melanoma using SER-401, an orally delivered Firmicutesenriched spore formulation. Fecal microbiota signatures were characterized at baseline; patients were stratified by high versus low Ruminococcaceae abundance prior to randomization to the SER-401 arm (oral vancomycin-preconditioning/SER-401 alone/nivolumab + SER-401), versus the placebo arm [placebo antibiotic/placebo microbiome modulation (PMM)/nivolumab + PMM (NCT03817125)]. Analysis of 14 accrued patients demonstrated that treatment with SER-401 + nivolumab was safe, with an overall response rate of 25% in the SER-401 arm and 67% in the placebo arm (though the study was underpowered related to poor accrual during the COVID-19 pandemic). Translational analyses demonstrated that vancomycin preconditioning was associated with the disruption of the gut microbiota and impaired immunity, with incomplete recovery at ICB administration (particularly in patients with high baseline Ruminococcaceae). These results have important implications for future microbiome modulation trials. Significance: This first-of-its-kind, placebo-controlled, randomized biomarker-driven microbiome modulation trial demonstrated that vancomycin + SER-401 and anti-PD-1 are safe in melanoma patients. Although limited by poor accrual during the pandemic, important insights were gained via translational analyses, suggesting that antibiotic preconditioning and interventional drug dosing regimens should be carefully considered when designing such trials.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
I.C. Glitza reports grants from BMS during the conduct of the study; grants, personal fees, and other support from Pfizer, personal fees from Novartis, grants from Merck, and personal fees from Midatech outside the submitted work. C.N. Spencer reports other support from the Parker Institute for Cancer Immunotherapy during the conduct of the study; personal fees from the Rare Cancer Research Foundation outside the submitted work; in addition, C.N. Spencer has a patent for improving responses to ICB therapy by enhancing the diversity and composition of the gut microbiome in patients with cancer licensed. J.R. Wortman reports other support from Seres Therapeutics outside the submitted work; in addition, J.R. Wortman has a patent for US20210361721A1 pending. J. Fairchild reports personal fees from the Parker Institute for Cancer Immunotherapy during the conduct of the study. C.B. Peterson reports grants from NIH/NCI CCSG 5P30CA016672 (Biostatistics Resource Group) during the conduct of the study. B. Weiner reports other support from Immuneering Corporation outside the submitted work. N. Hicks reports other support from Seres Therapeutics outside the submitted work. J. Aunins reports other support from the Parker Institute for Cancer Immunotherapy during the conduct of the study; other support from Seres Therapeutics, Inc. outside the submitted work. C. McChalicher reports employment and shareholder relationship with Seres Therapeutics. E. Walsh reports other support from Seres Therapeutics outside the submitted work. O. Hamid reports personal fees from Alkermes, Amgen, Beigene, Bioatla, Eisai, Roche Genentech, Georgiamune, GigaGen, GSK, GSK, Idera, Incyte, Instilbio, IO Bio, Iovance, Janssen, KSQ, Merck, Moderna, NGM, Obsidian, Sanofi, Seattle Genetics, Tempus, Vial Health, Zelluna, Bactonix, BMS, Immunocore, and Novartis, Pfizer, Regeneron during the conduct of the study; and contracted research for institution: Arcus, Aduro, Akeso, Amgen, Bioatla, BMS, Cytomx, Exelixis, Roche Genentech, GSK, Immunocore, Idera, Incyte, Iovance, Merck, Moderna, Merck Serono, Nextcure, Novartis, Pfizer, Regeneron, Seattle Genetics, Torque, Zelluna. P.A. Ott reports grants from Parker Institute during the conduct of the study; grants and personal fees from Bristol-Myers Squibb, Genentech, Merck, grants from Celldex, personal fees from Evaxion, Servier, Phio, and Pharmajet outside the submitted work. G.M. Boland reports grants from Olink Proteomics, Teiko Bio, Palleon Pharmaceuticals, personal fees from Iovance, Merck, Novartis, and Ankyra Therapeutics, and grants and personal fees from InterVenn Biosciences outside the submitted work. R.J. Sullivan reports grants and personal fees from Merck, personal fees from Novartis, Pfizer, Marengo, and Replimune outside the submitted work. N.J. Ajami reports other support from Seres Therapeutics and other support from Parker Institute for Cancer Immunotherapy during the conduct of the study. T. LaVallee reports grants and other support from PICI during the conduct of the study; personal fees from PICI outside the submitted work. M.R. Henn reports other support from the Parker Institute for Cancer Immunotherapy during the conduct of the study; personal fees and other support from Seres Therapeutics outside the submitted work. H.A. Tawbi reports grants and personal fees from Bristol-Myers Squibb, Merck, Novartis, grants from Genentech, personal fees from Pfizer, Iovance, Eisai, grants from Dragonfly, RAPT Therapeutics, personal fees from Karypharm, Jazz Pharmaceuticals, and Medicenna outside the submitted work. J.A. Wargo reports other support from Parker Institute for Cancer Immunotherapy and Seres Therapeutics during the conduct of the study; other support from Gustave Roussy Cancer Center, EverImmune, OSE Immunotherapeutics, Micronoma, and Daiichi Sankyo outside the submitted work; in addition, J.A. Wargo has a patent for PCT/US17/53.717 issued. No disclosures were reported by the other authors.
Figures

References
-
- Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 2016;315:1600–9. - PubMed
-
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–32. - PubMed
-
- Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob J-J, Rutkowski P, Cowey CL, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 2018;19:1480–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

