The PARTNER trial of neoadjuvant olaparib with chemotherapy in triple-negative breast cancer
- PMID: 38588696
- PMCID: PMC11136660
- DOI: 10.1038/s41586-024-07384-2
The PARTNER trial of neoadjuvant olaparib with chemotherapy in triple-negative breast cancer
Abstract
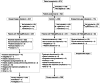
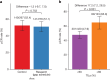
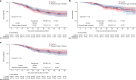
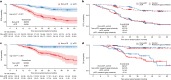
PARTNER is a prospective, phase II-III, randomized controlled clinical trial that recruited patients with triple-negative breast cancer1,2, who were germline BRCA1 and BRCA2 wild type3. Here we report the results of the trial. Patients (n = 559) were randomized on a 1:1 basis to receive neoadjuvant carboplatin-paclitaxel with or without 150 mg olaparib twice daily, on days 3 to 14, of each of four cycles (gap schedule olaparib, research arm) followed by three cycles of anthracycline-based chemotherapy before surgery. The primary end point was pathologic complete response (pCR)4, and secondary end points included event-free survival (EFS) and overall survival (OS)5. pCR was achieved in 51% of patients in the research arm and 52% in the control arm (P = 0.753). Estimated EFS at 36 months in the research and control arms was 80% and 79% (log-rank P > 0.9), respectively; OS was 90% and 87.2% (log-rank P = 0.8), respectively. In patients with pCR, estimated EFS at 36 months was 90%, and in those with non-pCR it was 70% (log-rank P < 0.001), and OS was 96% and 83% (log-rank P < 0.001), respectively. Neoadjuvant olaparib did not improve pCR rates, EFS or OS when added to carboplatin-paclitaxel and anthracycline-based chemotherapy in patients with triple-negative breast cancer who were germline BRCA1 and BRCA2 wild type. ClinicalTrials.gov ID: NCT03150576 .
© 2024. The Author(s).
Conflict of interest statement
The authors declare competing financial interests in the form of research grants and honoraria for lectures. The funders of the research grants and honoraria had no role in the study design, data collection, analysis, interpretation or writing of the manuscript. J.E.A. reports honoraria, conference attendance travel support and a grant from AstraZeneca; and honoraria from Esai and Pfizer for lectures. M.M. reports shares in AstraZeneca. M.B.M. reports advisory board membership of Roche, Pfizer, MSD, Daiichi Sankyo, Gilead, AstraZeneca, Novartis, the Menarini Group, Genomic Health (Precision Medicine) and Seagen; speaker honoraria from Roche, BMS, Seagen, Pfizer, Daiichi Sankyo, AstraZeneca, Lilly, MSD, Genomic Health (Precision Medicine), Eisai and Novartis; and meeting expenses from Roche, Eli Lilly, Novartis and MSD. R.R.R. reports honoraria from Daiichi Sankyo, AstraZeneca, Novartis and Pfizer; membership of advisory boards for Daiichi Sankyo, Eli Lilly, Pfizer and AstraZeneca; and travel and conference attendance from BMS, Pfizer and Roche. P.C.S. reports that their partner is employed by AstraZeneca. N.C.L. reports shares in AstraZeneca. A.C.A. reports research funding paid to their institution from AstraZeneca; conference fees and travel expenses from Roche and Novartis; conference fees from MSD; membership of Roche and AstraZeneca advisory boards; and a grant for an educational project from Gilead. E.R.C. reports honoraria from AstraZeneca, Eli Lilly, Novartis, Pfizer and Roche; membership of advisory boards for AstraZeneca, Eli Lilly, Pfizer, Menarini Stemline UK and Novartis; consultancy for Pfizer; conference fees, travel and accommodation from Roche and Novartis; an educational grant from Daiichi Sankyo; and research funding and support from SECA and AstraZeneca. E.P. reports honoraria from Roche, Novartis and AstraZeneca. The remaining authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

