Cordycepin Enhanced Therapeutic Potential of Gemcitabine against Cholangiocarcinoma via Downregulating Cancer Stem-Like Properties
- PMID: 38589021
- PMCID: PMC11063483
- DOI: 10.4062/biomolther.2023.198
Cordycepin Enhanced Therapeutic Potential of Gemcitabine against Cholangiocarcinoma via Downregulating Cancer Stem-Like Properties
Abstract
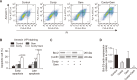
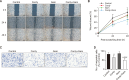
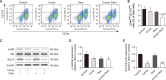
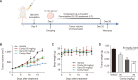
Cordycepin, a valuable bioactive component isolated from Cordyceps militaris, has been reported to possess anti-cancer potential and the property to enhance the effects of chemotherapeutic agents in various types of cancers. However, the ability of cordycepin to chemosensitize cholangiocarcinoma (CCA) cells to gemcitabine has not yet been evaluated. The current study was performed to evaluate the above, and the mechanisms associated with it. The study analyzed the effects of cordycepin in combination with gemcitabine on the cancer stem-like properties of the CCA SNU478 cell line, including its anti-apoptotic, migratory, and antioxidant effects. In addition, the combination of cordycepin and gemcitabine was evaluated in the CCA xenograft model. The cordycepin treatment significantly decreased SNU478 cell viability and, in combination with gemcitabine, additively reduced cell viability. The cordycepin and gemcitabine co-treatment significantly increased the Annexin V+ population and downregulated B-cell lymphoma 2 (Bcl-2) expression, suggesting that the decreased cell viability in the cordycepin+gemcitabine group may result from an increase in apoptotic death. In addition, the cordycepin and gemcitabine co-treatment significantly reduced the migratory ability of SNU478 cells in the wound healing and trans-well migration assays. It was observed that the cordycepin and gemcitabine cotreatment reduced the CD44highCD133high population in SNU478 cells and the expression level of sex determining region Y-box 2 (Sox-2), indicating the downregulation of the cancer stem-like population. Cordycepin also enhanced oxidative damage mediated by gemcitabine in MitoSOX staining associated with the upregulated Kelch like ECH Associated Protein 1 (Keap1)/nuclear factor erythroid 2-related factor 2 (Nrf2) expression ratio. In the SNU478 xenograft model, co-administration of cordycepin and gemcitabine additively delayed tumor growth. These results indicate that cordycepin potentiates the chemotherapeutic property of gemcitabine against CCA, which results from the downregulation of its cancer-stem-like properties. Hence, the combination therapy of cordycepin and gemcitabine may be a promising therapeutic strategy in the treatment of CCA.
Keywords: Cancer stem cell; Chemoresistance; Cholangiocarcinoma; Cordycepin; Gemcitabine.
Conflict of interest statement
The authors do not have any conflict of interest to declare.
Figures



Similar articles
-
Amurensin G Sensitized Cholangiocarcinoma to the Anti-Cancer Effect of Gemcitabine via the Downregulation of Cancer Stem-like Properties.Nutrients. 2023 Dec 25;16(1):73. doi: 10.3390/nu16010073. Nutrients. 2023. PMID: 38201903 Free PMC article.
-
Cordycepin Sensitizes Cholangiocarcinoma Cells to Be Killed by Natural Killer-92 (NK-92) Cells.Molecules. 2021 Oct 1;26(19):5973. doi: 10.3390/molecules26195973. Molecules. 2021. PMID: 34641520 Free PMC article.
-
GDC-0980 (apitolisib) treatment with gemcitabine and/or cisplatin synergistically reduces cholangiocarcinoma cell growth by suppressing the PI3K/Akt/mTOR pathway.Biochem Biophys Res Commun. 2020 Sep 3;529(4):1242-1248. doi: 10.1016/j.bbrc.2020.06.011. Epub 2020 Aug 8. Biochem Biophys Res Commun. 2020. PMID: 32819590
-
Atorvastatin Augments Gemcitabine-Mediated Anti-Cancer Effects by Inhibiting Yes-Associated Protein in Human Cholangiocarcinoma Cells.Int J Mol Sci. 2020 Oct 14;21(20):7588. doi: 10.3390/ijms21207588. Int J Mol Sci. 2020. PMID: 33066548 Free PMC article.
-
Optimal conditions for cordycepin production in surface liquid-cultured Cordyceps militaris treated with porcine liver extracts for suppression of oral cancer.J Food Drug Anal. 2018 Jan;26(1):135-144. doi: 10.1016/j.jfda.2016.11.021. Epub 2017 Feb 16. J Food Drug Anal. 2018. PMID: 29389548 Free PMC article.
References
-
- Alabraba E., Joshi H., Bird N., Griffin R., Sturgess R., Stern N., Sieberhagen C., Cross T., Camenzuli A., Davis R., Evans J., O'Grady E., Palmer D., Diaz-Nieto R., Fenwick S., Poston G., Malik H. Increased multimodality treatment options has improved survival for Hepatocellular carcinoma but poor survival for biliary tract cancers remains unchanged. Eur. J. Surg. Oncol. 2019;45:1660–1667. doi: 10.1016/j.ejso.2019.04.002. - DOI - PubMed
-
- Ashraf S. A., Elkhalifa A. E. O., Siddiqui A. J., Patel M., Awadelkareem A. M., Snoussi M., Ashraf M. S., Adnan M., Hadi S. Cordycepin for health and wellbeing: a potent bioactive metabolite of an entomopathogenic cordyceps medicinal fungus and its nutraceutical and therapeutic potential. Molecules. 2020;25:2735. doi: 10.3390/molecules25122735.4f0a4aef061c4e2985c3c4eb8e1104a7 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

