Impaired Cortical Tracking of Speech in Children with Developmental Language Disorder
- PMID: 38589232
- PMCID: PMC11140678
- DOI: 10.1523/JNEUROSCI.2048-23.2024
Impaired Cortical Tracking of Speech in Children with Developmental Language Disorder
Erratum in
-
Erratum: Nora et al., "Impaired Cortical Tracking of Speech in Children with Developmental Language Disorder".J Neurosci. 2024 Jul 31;44(31):e1260242024. doi: 10.1523/JNEUROSCI.1260-24.2024. J Neurosci. 2024. PMID: 39084939 Free PMC article. No abstract available.
Abstract
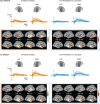
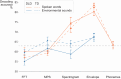
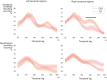
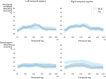
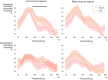
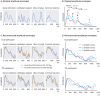
In developmental language disorder (DLD), learning to comprehend and express oneself with spoken language is impaired, but the reason for this remains unknown. Using millisecond-scale magnetoencephalography recordings combined with machine learning models, we investigated whether the possible neural basis of this disruption lies in poor cortical tracking of speech. The stimuli were common spoken Finnish words (e.g., dog, car, hammer) and sounds with corresponding meanings (e.g., dog bark, car engine, hammering). In both children with DLD (10 boys and 7 girls) and typically developing (TD) control children (14 boys and 3 girls), aged 10-15 years, the cortical activation to spoken words was best modeled as time-locked to the unfolding speech input at ∼100 ms latency between sound and cortical activation. Amplitude envelope (amplitude changes) and spectrogram (detailed time-varying spectral content) of the spoken words, but not other sounds, were very successfully decoded based on time-locked brain responses in bilateral temporal areas; based on the cortical responses, the models could tell at ∼75-85% accuracy which of the two sounds had been presented to the participant. However, the cortical representation of the amplitude envelope information was poorer in children with DLD compared with TD children at longer latencies (at ∼200-300 ms lag). We interpret this effect as reflecting poorer retention of acoustic-phonetic information in short-term memory. This impaired tracking could potentially affect the processing and learning of words as well as continuous speech. The present results offer an explanation for the problems in language comprehension and acquisition in DLD.
Keywords: development; developmental language disorder; machine learning; magnetoencephalography; speech processing.
Copyright © 2024 Nora et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Attout L, Grégoire C, Majerus S (2020) How robust is the link between working memory for serial order and lexical skills in children? Cogn Dev 53:100854. 10.1016/j.cogdev.2020.100854 - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
