Stress increases hepatic release of lipocalin 2 which contributes to anxiety-like behavior in mice
- PMID: 38589429
- PMCID: PMC11001612
- DOI: 10.1038/s41467-024-47266-9
Stress increases hepatic release of lipocalin 2 which contributes to anxiety-like behavior in mice
Abstract
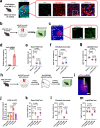
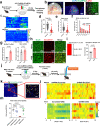
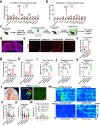
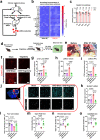
Chronic stress induces anxiety disorders via both neural pathways and circulating factors. Although many studies have elucidated the neural circuits involved in stress-coping behaviors, the origin and regulatory mechanism of peripheral cytokines in behavioural regulation under stress conditions are not fully understood. Here, we identified a serum cytokine, lipocalin 2 (LCN2), that was upregulated in participants with anxiety disorders. Using a mouse model of chronic restraint stress (CRS), circulating LCN2 was found to be related to stress-induced anxiety-like behaviour via modulation of neural activity in the medial prefrontal cortex (mPFC). These results suggest that stress increases hepatic LCN2 via a neural pathway, leading to disrupted cortical functions and behaviour.
© 2024. The Author(s).
Conflict of interest statement
All authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

