A cyclical switch of gametogenic pathways in hybrids depends on the ploidy level
- PMID: 38589507
- PMCID: PMC11001910
- DOI: 10.1038/s42003-024-05948-6
A cyclical switch of gametogenic pathways in hybrids depends on the ploidy level
Abstract
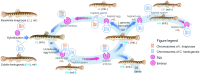
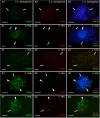
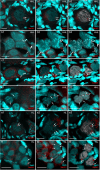
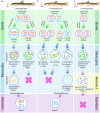
The cellular and molecular mechanisms governing sexual reproduction are conserved across eukaryotes. Nevertheless, hybridization can disrupt these mechanisms, leading to asexual reproduction, often accompanied by polyploidy. In this study, we investigate how ploidy level and ratio of parental genomes in hybrids affect their reproductive mode. We analyze the gametogenesis of sexual species and their diploid and triploid hybrids from the freshwater fish family Cobitidae, using newly developed cytogenetic markers. We find that diploid hybrid females possess oogonia and oocytes with original (diploid) and duplicated (tetraploid) ploidy. Diploid oocytes cannot progress beyond pachytene due to aberrant pairing. However, tetraploid oocytes, which emerge after premeiotic genome endoreplication, exhibit normal pairing and result in diploid gametes. Triploid hybrid females possess diploid, triploid, and haploid oogonia and oocytes. Triploid and haploid oocytes cannot progress beyond pachytene checkpoint due to aberrant chromosome pairing, while diploid oocytes have normal pairing in meiosis, resulting in haploid gametes. Diploid oocytes emerge after premeiotic elimination of a single-copied genome. Triploid hybrid males are sterile due to aberrant pairing and the failure of chromosomal segregation during meiotic divisions. Thus, changes in ploidy and genome dosage may lead to cyclical alteration of gametogenic pathways in hybrids.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Coyne, J. A., Orr, H. A., Coyne, J. A. & Orr, H. A. Speciation (Oxford University Press, 2004).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

