Maternal diabetes and risk of attention-deficit/hyperactivity disorder in offspring in a multinational cohort of 3.6 million mother-child pairs
- PMID: 38589601
- PMCID: PMC11108779
- DOI: 10.1038/s41591-024-02917-8
Maternal diabetes and risk of attention-deficit/hyperactivity disorder in offspring in a multinational cohort of 3.6 million mother-child pairs
Abstract
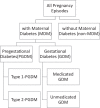
Previous studies report an association between maternal diabetes mellitus (MDM) and attention-deficit/hyperactivity disorder (ADHD), often overlooking unmeasured confounders such as shared genetics and environmental factors. We therefore conducted a multinational cohort study with linked mother-child pairs data in Hong Kong, New Zealand, Taiwan, Finland, Iceland, Norway and Sweden to evaluate associations between different MDM (any MDM, gestational diabetes mellitus (GDM) and pregestational diabetes mellitus (PGDM)) and ADHD using Cox proportional hazards regression. We included over 3.6 million mother-child pairs between 2001 and 2014 with follow-up until 2020. Children who were born to mothers with any type of diabetes during pregnancy had a higher risk of ADHD than unexposed children (pooled hazard ratio (HR) = 1.16, 95% confidence interval (CI) = 1.08-1.24). Higher risks of ADHD were also observed for both GDM (pooled HR = 1.10, 95% CI = 1.04-1.17) and PGDM (pooled HR = 1.39, 95% CI = 1.25-1.55). However, siblings with discordant exposure to GDM in pregnancy had similar risks of ADHD (pooled HR = 1.05, 95% CI = 0.94-1.17), suggesting potential confounding by unmeasured, shared familial factors. Our findings indicate that there is a small-to-moderate association between MDM and ADHD, whereas the association between GDM and ADHD is unlikely to be causal. This finding contrast with previous studies, which reported substantially higher risk estimates, and underscores the need to reevaluate the precise roles of hyperglycemia and genetic factors in the relationship between MDM and ADHD.
© 2024. The Author(s).
Conflict of interest statement
A.H.Y.C. receives research funding from Health Research Council NZ, Oakley Mental Health Foundation, NZ Pharmacy Education and Research Foundation, Ministry of Health, World Health Organisation and is the recipient of fellowships from the Robert Irwin Foundation and Auckland Medical Research Foundation, unrelated to the submitted work. She is also affiliated with the Asthma UK Centre of Applied Research and is on the Board of Asthma NZ and Pharmacy Council of New Zealand. D.C. reports research funding outside the submitted work from the Australian National Health and Medical Research Council, speaker’s fees and honoraria from Novartis, Medice, Servier and Shire/Takeda and royalties from Oxford University Press and Cambridge University Press in the past 3 years. M.G. and M.K.L. report that they received grants from the Innovative Medicines Initiative (Building an ecosystem for better monitoring and communicating the safety of medicines’ use in pregnancy and breastfeeding: validated and regulatory endorsed workflows for fast, optimized evidence generation, IMI ConcePTION, grant agreement number 821520) while conducting the study. J.H. receives research funding from the Health Research Council NZ, Lotteries Health Research (New Zealand), New Zealand Ministry of Health and New Zealand Health Quality Safety Commission unrelated to the submitted work. P.I. reports research funding from the Hong Kong Research Grants Council, Health and Medical Research Fund and Hong Kong Jockey Club Charities Trust. Ø.K. reports participation in regulator-mandated post-authorization safety studies (PASS) of drugs with no relation to the work reported in this paper. The studies are funded by Leo Pharma and Novo Nordisk, with funds paid to the institution where he is employed (no personal fees). W.C.Y.L. reports research grants from Diabetes UK, AIR@InnoHK administered by Innovation and Technology Commission outside the submitted work. J.R. and C.E.C. are employees of the Centre for Pharmacoepidemiology at Karolinska Institutet, which receives funding from pharmaceutical companies and regulatory authorities for drug safety/utilization studies, unrelated to the submitted work. E.S. receives research funding from the UK National Institute of Health Research, United Kingdom Research and Innovation, and the European Innovative Medicines Initiative. E.C.-C.L. reports research funding outside the submitted work from Amgen, Pfizer, Sanofi, Takeda, Roche, IQVIA. H.Z. was supported by a UNSW Scientia Program Award and reports grants from the European Union Horizon 2020, Australian National Health and Medical Research Council (NHMRC), Icelandic Centre for Research, NordForsk Nordic Council of Ministers during the conduct of this study. K.K.C.M. is the recipient of the CW Maplethorpe Fellowship, reports grants from the European Union Horizon 2020, the UK National Institute of Health Research and the Hong Kong Research Grant Council, Hong Kong Innovation and Technology Commission, and reports personal fees from IQVIA unrelated to the submitted work. I.C.K.W. received research grants from Amgen, Janssen, GSK, Novartis, Pfizer, Bayer and Bristol-Myers Squibb and Takeda, Institute for Health Research in England, European Commission, National Health and Medical Research Council in Australia, The European Union’s Seventh Framework Programme for research, technological development, Research Grants Council Hong Kong and Health and Medical Research Fund Hong Kong; consulting fees from IQVIA and World Health Organization; payment for expert testimony for Appeal Court in Hong Kong; serves on advisory committees for Member of Pharmacy and Poisons Board; is a member of the Expert Committee on Clinical Events Assessment Following COVID-19 Immunization; is a member of the Advisory Panel on COVID-19 Vaccines of the Hong Kong Government; is the non-executive director of Jacobson Medical in Hong Kong; and is the founder and director of Therakind Limited (UK), Advance Data Analytics for Medical Science (ADAMS) Limited (HK), Asia Medicine Regulatory Affairs (AMERA) Services Limited and OCUS Innovation Limited (HK, Ireland and UK). A.Y.L.C. is supported by the AIR@innoHK programme of the Hong Kong Innovation and Technology Commission. L.G., M.H.-C.H., L.J.K., R.A., T.B., J.M.C., W.C.L., T.-C.L., S.-C.S., K.C.B.T., K.T. and A.T. declare no competing interests.
Figures








References
MeSH terms
Grants and funding
- 17112020/Research Grants Council, University Grants Committee (RGC, UGC)
- 965381/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 844728/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 Marie Skłodowska-Curie Actions (H2020 Excellent Science - Marie Skłodowska-Curie Actions)
- 83539/NordForsk
- 273366, 262700/Norges Forskningsråd (Research Council of Norway)
LinkOut - more resources
Full Text Sources
Medical

