Structure of adenylyl cyclase 5 in complex with Gβγ offers insights into ADCY5-related dyskinesia
- PMID: 38589608
- PMCID: PMC11329361
- DOI: 10.1038/s41594-024-01263-0
Structure of adenylyl cyclase 5 in complex with Gβγ offers insights into ADCY5-related dyskinesia
Abstract
The nine different membrane-anchored adenylyl cyclase isoforms (AC1-9) in mammals are stimulated by the heterotrimeric G protein, Gαs, but their response to Gβγ regulation is isoform specific. In the present study, we report cryo-electron microscope structures of ligand-free AC5 in complex with Gβγ and a dimeric form of AC5 that could be involved in its regulation. Gβγ binds to a coiled-coil domain that links the AC transmembrane region to its catalytic core as well as to a region (C1b) that is known to be a hub for isoform-specific regulation. We confirmed the Gβγ interaction with both purified proteins and cell-based assays. Gain-of-function mutations in AC5 associated with human familial dyskinesia are located at the interface of AC5 with Gβγ and show reduced conditional activation by Gβγ, emphasizing the importance of the observed interaction for motor function in humans. We propose a molecular mechanism wherein Gβγ either prevents dimerization of AC5 or allosterically modulates the coiled-coil domain, and hence the catalytic core. As our mechanistic understanding of how individual AC isoforms are uniquely regulated is limited, studies such as this may provide new avenues for isoform-specific drug development.
© 2024. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests
The authors declare that they have no competing interests.
Figures

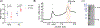
 ) or presence (
) or presence ( ) of 400 nM Gβ1γ2. Data presented are the mean ± SD. (B) The AC5–Gβγ complex was purified using anti-FLAG M2 affinity resin and injected onto a Superose 6 Increase 3.2/300 column equilibrated with 50 mM HEPES pH 8, 100 mM NaCl, 10 mM MgCl2, and 0.01 % LMNG. Peak fractions highlighted in pink were pooled, concentrated, and (C) analyzed by SDS-PAGE (note that Gγ2 runs off the gel).
) of 400 nM Gβ1γ2. Data presented are the mean ± SD. (B) The AC5–Gβγ complex was purified using anti-FLAG M2 affinity resin and injected onto a Superose 6 Increase 3.2/300 column equilibrated with 50 mM HEPES pH 8, 100 mM NaCl, 10 mM MgCl2, and 0.01 % LMNG. Peak fractions highlighted in pink were pooled, concentrated, and (C) analyzed by SDS-PAGE (note that Gγ2 runs off the gel).




 ) or GDN (
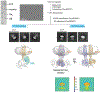
) or GDN ( ) was determined in the presence of 10 mM MgCl2 and 0.5 mM ATP. (C) Gαs and (D) Gβγ dose-response curve for AC5 reconstituted in LMNG (
) was determined in the presence of 10 mM MgCl2 and 0.5 mM ATP. (C) Gαs and (D) Gβγ dose-response curve for AC5 reconstituted in LMNG ( ) or GDN (
) or GDN ( ). AC5 activity was determined in the presence of 10 mM MgCl2, 0.5 mM ATP at the indicated Gαs concentration or 100 nM Gαs·GTPγS at the indicated Gβγ concentrations, with data presented as mean ± SD (n=3).
). AC5 activity was determined in the presence of 10 mM MgCl2, 0.5 mM ATP at the indicated Gαs concentration or 100 nM Gαs·GTPγS at the indicated Gβγ concentrations, with data presented as mean ± SD (n=3).





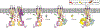
References
-
- Krupinski J et al. Adenylyl cyclase amino acid sequence: possible channel- or transporter-like structure. Science 244, 1558–64 (1989). - PubMed
-
- Crossthwaite AJ et al. The cytosolic domains of Ca2+-sensitive adenylyl cyclases dictate their targeting to plasma membrane lipid rafts. J Biol Chem 280, 6380–91 (2005). - PubMed
-
- Gu C & Cooper DM Calmodulin-binding sites on adenylyl cyclase type VIII. J Biol Chem 274, 8012–21 (1999). - PubMed
Methods-only references
-
- Lee E, Linder ME & Gilman AG Expression of G-protein alpha subunits in Escherichia coli. Methods Enzymol 237, 146–64 (1994). - PubMed
-
- Dessauer CW Kinetic analysis of the action of P-site analogs. Methods Enzymol 345, 112–26 (2002). - PubMed
-
- Punjani A, Rubinstein JL, Fleet DJ & Brubaker MA cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat Methods 14, 290–296 (2017). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

