Neutrophil extracellular traps promote acetaminophen-induced acute liver injury in mice via AIM2
- PMID: 38589685
- PMCID: PMC11272772
- DOI: 10.1038/s41401-024-01239-2
Neutrophil extracellular traps promote acetaminophen-induced acute liver injury in mice via AIM2
Abstract
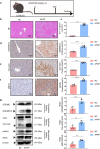
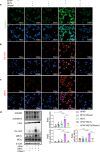
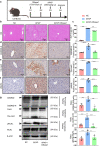
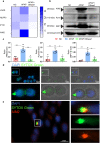
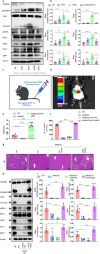
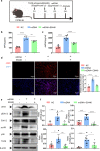
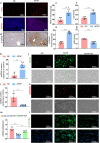
Excessive acetaminophen (APAP) can induce neutrophil activation and hepatocyte death. Along with hepatocyte dysfunction and death, NETosis (a form of neutrophil-associated inflammation) plays a vital role in the progression of acute liver injury (ALI) induced by APAP overdose. It has been shown that activated neutrophils tend to migrate towards the site of injury and participate in inflammatory processes via formation of neutrophil extracellular traps (NETs). In this study we investigated whether NETs were involved in hepatocyte injury and contributed to APAP-induced ALI progression. ALI mouse model was established by injecting overdose (350 mg/kg) of APAP. After 24 h, blood and livers were harvested for analyses. We showed that excessive APAP induced multiple programmed cell deaths of hepatocytes including pyroptosis, apoptosis and necroptosis, accompanied by significantly increased NETs markers (MPO, citH3) in the liver tissue and serum. Preinjection of DNase1 (10 U, i.p.) for two consecutive days significantly inhibited NETs formation, reduced PANoptosis and consequently alleviated excessive APAP-induced ALI. In order to clarify the communication between hepatocytes and neutrophils, we induced NETs formation in isolated neutrophils, and treated HepaRG cells with NETs. We found that NETs treatment markedly increased the activation of GSDMD, caspase-3 and MLKL, while pre-treatment with DNase1 down-regulated the expression of these proteins. Knockdown of AIM2 (a cytosolic innate immune receptor) abolished NETs-induced PANoptosis in HepaRG cells. Furthermore, excessive APAP-associated ALI was significantly attenuated in AIM2KO mice, and PANoptosis occurred less frequently. Upon restoring AIM2 expression in AIM2KO mice using AAV9 virus, both hepatic injury and PANoptosis was aggravated. In addition, we demonstrated that excessive APAP stimulated mtROS production and mitochondrial DNA (mtDNA) leakage, and mtDNA activated the TLR9 pathway to promote NETs formation. Our results uncover a novel mechanism of NETs and PANoptosis in APAP-associated ALI, which might serve as a therapeutic target.
Keywords: AIM2; APAP; NETs; PANoptosis; TLR9; acute liver injury.
© 2024. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

