Assessing antiviral treatment efficacy and risk factors for severe COVID-19 in kidney transplant recipients during the Omicron subvariant-dominant period: a retrospective study
- PMID: 38589827
- PMCID: PMC11000285
- DOI: 10.1186/s12882-024-03561-7
Assessing antiviral treatment efficacy and risk factors for severe COVID-19 in kidney transplant recipients during the Omicron subvariant-dominant period: a retrospective study
Abstract
Background: Kidney transplant recipients (KTRs) are at risk of severe coronavirus disease 2019 (COVID-19), and even now that Omicron subvariants have become dominant, cases of severe disease are certain to occur. The aims of this retrospective study were to evaluate the efficacy of antiviral treatment for COVID-19 and to identify risk factors for severe disease in KTRs during Omicron subvariant-dominant periods.
Methods: A total of 65 KTRs diagnosed with COVID-19 who received antiviral treatment between July 2022 and September 2023 were analyzed. Mild cases received oral molnupiravir (MP) as outpatient therapy, while moderate or worse cases received intravenous remdesivir (RDV) as inpatient therapy. In principle, mycophenolate mofetil was withdrawn and switched to everolimus. We investigated the efficacy of antiviral treatment and compared the clinical parameters of mild/moderate and severe/critical cases to identify risk factors for severe COVID-19.
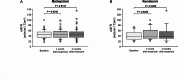
Results: Among 65 cases, 49 were mild, 6 were moderate, 9 were severe, and 1 was of critical severity. MP was administered to 57 cases; 49 (86%) improved and 8 (14%) progressed. RDV was administered to 16 cases; 14 (87%) improved and 2 (13%) progressed. Seventeen (26%) cases required hospitalization, and none died. Comparisons of the severe/critical group (n = 10) with the mild/moderate group (n = 55) demonstrated that the severe/critical group had a significantly higher median age (64 vs. 53 years, respectively; p = 0.0252), prevalence of diabetes (70% vs. 22%, respectively; p = 0.0047) and overweight/obesity (40% vs. 11%, respectively; p = 0.0393), as well as a significantly longer median time from symptom onset to initial antiviral therapy (3 days vs. 1 day, respectively; p = 0.0026). Multivariate analysis showed that a longer time from symptom onset to initial antiviral treatment was an independent risk factor for severe COVID-19 (p = 0.0196, odds ratio 1.625, 95% confidence interval 1.081-2.441).
Conclusion: These findings suggest that a longer time from symptom onset to initial antiviral treatment is associated with a higher risk of severe COVID-19 in KTRs. Initiating antiviral treatment as early as possible is crucial for preventing severe outcomes; this represents a valuable insight into COVID-19 management in KTRs.
Keywords: COVID-19; Kidney transplant recipients; Molnupiravir; Remdesivir.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Menni C, Valdes AM, Polidori L, Antonelli M, Penamakuri S, Nogal A, Louca P, May A, Figueiredo JC, Hu C, et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. 2022;399(10335):1618–24. doi: 10.1016/S0140-6736(22)00327-0. - DOI - PMC - PubMed
-
- Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, Martín-Quirós A, Caraco Y, Williams-Diaz A, Brown ML, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–20. doi: 10.1056/NEJMoa2116044. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

