Ibrutinib disrupts blood-tumor barrier integrity and prolongs survival in rodent glioma model
- PMID: 38589905
- PMCID: PMC11003129
- DOI: 10.1186/s40478-024-01763-6
Ibrutinib disrupts blood-tumor barrier integrity and prolongs survival in rodent glioma model
Abstract
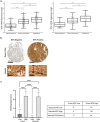
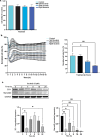
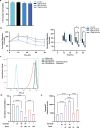
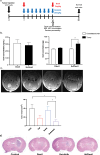
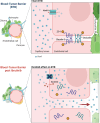
In malignant glioma, cytotoxic drugs are often inhibited from accessing the tumor site due to the blood-tumor barrier (BTB). Ibrutinib, FDA-approved lymphoma agent, inhibits Bruton tyrosine kinase (BTK) and has previously been shown to independently impair aortic endothelial adhesion and increase rodent glioma model survival in combination with cytotoxic therapy. Yet additional research is required to understand ibrutinib's effect on BTB function. In this study, we detail baseline BTK expression in glioma cells and its surrounding vasculature, then measure endothelial junctional expression/function changes with varied ibrutinib doses in vitro. Rat glioma cells and rodent glioma models were treated with ibrutinib alone (1-10 µM and 25 mg/kg) and in combination with doxil (10-100 µM and 3 mg/kg) to assess additive effects on viability, drug concentrations, tumor volume, endothelial junctional expression and survival. We found that ibrutinib, in a dose-dependent manner, decreased brain endothelial cell-cell adhesion over 24 h, without affecting endothelial cell viability (p < 0.005). Expression of tight junction gene and protein expression was decreased maximally 4 h after administration, along with inhibition of efflux transporter, ABCB1, activity. We demonstrated an additive effect of ibrutinib with doxil on rat glioma cells, as seen by a significant reduction in cell viability (p < 0.001) and increased CNS doxil concentration in the brain (56 ng/mL doxil alone vs. 74.6 ng/mL combination, p < 0.05). Finally, Ibrutinib, combined with doxil, prolonged median survival in rodent glioma models (27 vs. 16 days, p < 0.0001) with brain imaging showing a - 53% versus - 75% volume change with doxil alone versus combination therapy (p < 0.05). These findings indicate ibrutinib's ability to increase brain endothelial permeability via junctional disruption and efflux inhibition, to increase BTB drug entry and prolong rodent glioma model survival. Our results motivate the need to identify other BTB modifiers, all with the intent of improving survival and reducing systemic toxicities.
Keywords: Blood-tumor barrier; Doxil; Endothelial cells; Glioma; Ibrutinib.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Angulin-1/LSR inhibition transiently disrupts the blood-tumor barrier to enhance doxil permeability and impair malignant glioma progression.bioRxiv [Preprint]. 2025 Aug 2:2025.07.31.667901. doi: 10.1101/2025.07.31.667901. bioRxiv. 2025. PMID: 40766530 Free PMC article. Preprint.
-
Foetal haemoglobin inducers for reducing blood transfusion in non-transfusion-dependent beta-thalassaemias.Cochrane Database Syst Rev. 2023 Jan 13;1(1):CD013767. doi: 10.1002/14651858.CD013767.pub2. Cochrane Database Syst Rev. 2023. PMID: 36637054 Free PMC article.
-
Corticosteroids for the treatment of Duchenne muscular dystrophy.Cochrane Database Syst Rev. 2016 May 5;2016(5):CD003725. doi: 10.1002/14651858.CD003725.pub4. Cochrane Database Syst Rev. 2016. PMID: 27149418 Free PMC article.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.Health Technol Assess. 2007 Nov;11(45):iii-iv, ix-221. doi: 10.3310/hta11450. Health Technol Assess. 2007. PMID: 17999840
Cited by
-
Tight junction proteins in glial tumors development and progression.Front Cell Neurosci. 2025 Feb 3;19:1541885. doi: 10.3389/fncel.2025.1541885. eCollection 2025. Front Cell Neurosci. 2025. PMID: 39963115 Free PMC article. Review.
-
The transformative potential of mRNA vaccines for glioblastoma and human cancer: technological advances and translation to clinical trials.Front Oncol. 2024 Sep 27;14:1454370. doi: 10.3389/fonc.2024.1454370. eCollection 2024. Front Oncol. 2024. PMID: 39399167 Free PMC article. Review.
-
Angulin-1/LSR inhibition transiently disrupts the blood-tumor barrier to enhance doxil permeability and impair malignant glioma progression.bioRxiv [Preprint]. 2025 Aug 2:2025.07.31.667901. doi: 10.1101/2025.07.31.667901. bioRxiv. 2025. PMID: 40766530 Free PMC article. Preprint.
References
-
- van Tellingen O, Yetkin-Arik B, de Gooijer MC, Wesseling P, Wurdinger T, de Vries HE (2015) Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist Updates Rev Comment Antimicrob Anticancer Chemotherapy 19:1–12 - PubMed
-
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ (2010) Structure and function of the blood-brain barrier. Neurobiol Dis 37(1):13–25 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

