OpenSAFELY: The impact of COVID-19 on azathioprine, leflunomide and methotrexate monitoring, and factors associated with change in monitoring rate
- PMID: 38589944
- PMCID: PMC7616619
- DOI: 10.1111/bcp.16062
OpenSAFELY: The impact of COVID-19 on azathioprine, leflunomide and methotrexate monitoring, and factors associated with change in monitoring rate
Abstract
Aims: The COVID-19 pandemic created unprecedented pressure on healthcare services. This study investigates whether disease-modifying antirheumatic drug (DMARD) safety monitoring was affected during the COVID-19 pandemic.
Methods: A population-based cohort study was conducted using the OpenSAFELY platform to access electronic health record data from 24.2 million patients registered at general practices using TPP's SystmOne software. Patients were included for further analysis if prescribed azathioprine, leflunomide or methotrexate between November 2019 and July 2022. Outcomes were assessed as monthly trends and variation between various sociodemographic and clinical groups for adherence with standard safety monitoring recommendations.
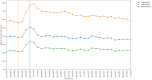
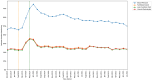
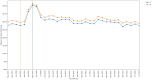
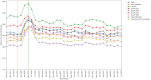
Results: An acute increase in the rate of missed monitoring occurred across the study population (+12.4 percentage points) when lockdown measures were implemented in March 2020. This increase was more pronounced for some patient groups (70-79 year-olds: +13.7 percentage points; females: +12.8 percentage points), regions (North West: +17.0 percentage points), medications (leflunomide: +20.7 percentage points) and monitoring tests (blood pressure: +24.5 percentage points). Missed monitoring rates decreased substantially for all groups by July 2022. Consistent differences were observed in overall missed monitoring rates between several groups throughout the study.
Conclusion: DMARD monitoring rates temporarily deteriorated during the COVID-19 pandemic. Deterioration coincided with the onset of lockdown measures, with monitoring rates recovering rapidly as lockdown measures were eased. Differences observed in monitoring rates between medications, tests, regions and patient groups highlight opportunities to tackle potential inequalities in the provision or uptake of monitoring services. Further research should evaluate the causes of the differences identified between groups.
Keywords: COVID‐19; antirheumatic agents; azathioprine; electronic health records; general practice; leflunomide; methotrexate.
© 2024 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form at
Figures


References
-
- National Institute for Health and Care Excellence .DMARDs;2022. https://cks.nice.org.uk/topics/dmards/
-
- BNF content published by NICE [Internet]; 2022. https://bnf.nice.org.uk/
MeSH terms
Substances
Grants and funding
- 222097/WT_/Wellcome Trust/United Kingdom
- MR/W016729/1/MRC_/Medical Research Council/United Kingdom
- MR/V015737/1/MRC_/Medical Research Council/United Kingdom
- COV-LT-0009/DH_/Department of Health/United Kingdom
- MC_PC_20030/MRC_/Medical Research Council/United Kingdom
- MC_PC_20058/MRC_/Medical Research Council/United Kingdom
- COV-LT2-0073/National Institute for Health and Care Research
- 222097/Z/20/Z/WT_/Wellcome Trust/United Kingdom
- HDRUK2021.000/Health Data Research UK
- MC_PC_20058/UK Research and Innovation
- NIHR135559/National Institute for Health and Care Research
- MC_PC-20059/MRC_/Medical Research Council/United Kingdom
- 2021.0157/Health Data Research UK
- COV-LT2-0073/DH_/Department of Health/United Kingdom
- MC_PC_20059/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Medical

