Canakinumab treatment real world evidence in 3 monogenic periodic fever syndromes in 2009-2022: an interim analysis using the French JIR cohort database
- PMID: 38589954
- PMCID: PMC11000371
- DOI: 10.1186/s13075-024-03316-7
Canakinumab treatment real world evidence in 3 monogenic periodic fever syndromes in 2009-2022: an interim analysis using the French JIR cohort database
Abstract
Background: Our study aimed to provide real-world evidence on the treatment patterns, effectiveness and safety of canakinumab in France in Familial Mediterranean Fever (FMF), Mevalonate Kinase Deficiency (MKD), and Tumor necrosis factor Receptor Associated Periodic Syndrome (TRAPS).
Methods: This study used the JIR cohort, a multicentre international registry created in 2013 to collect data on patients with juvenile inflammatory rheumatic diseases. French patients diagnosed with FMF, MKD or TRAPS and treated with canakinumab were included in this study.
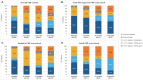
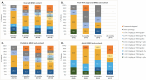
Results: 31 FMF, 26 MKD and 7 TRAPS patients received canakinumab during the study period. Most of them initiated canakinumab at the recommended dose of 2 mg/kg or 150 mg, but less than half of FMF and MKD patients initiated it at the recommended frequency (every 4 weeks). Two years after initiation, the rate of patients still on treatment was 78.1% in FMF, 73.7% in MKD, and 85.7% in TRAPS patients. While the dose per injection remained globally the same over the course of the treatment, some adjustments of the dose intervals were observed. Six patients had a severe adverse event reported. Of those, three were possibly related to canakinumab.
Conclusion: This interim analysis showed a good maintenance of canakinumab treatment 2 years after initiation and confirmed its safety profile in real-life practice in France in patients diagnosed with FMF, MKD and TRAPS. The high variety of dose and interval combinations observed in canakinumab treated patients let suppose that physicians adapt the posology to individual situations rather than a fixed treatment plan.
Keywords: Autoinflammatory diseases; Canakinumab; FMF; JIR cohort; MKD; Real-world-evidence; Safety; TRAPS; Treatment patterns.
© 2024. The Author(s).
Conflict of interest statement
IKP has received consulting fees, speaking fees and/or honoraria Novartis, SOBI, LFB, Abbvie, Pfizer, Chugai, Amgen, Zydus. SG has received consulting fees, speaking fees and/or honoraria from Novartis and SOBI. AB has received research grant from Boehringer Ingelheim and Merck Serono and consulting fees from Novartis, Abbvie, GSK, Roche Chugai. MJ is an employee of Novartis. MP is an employee of I&M STAT working for Novartis. LL has received consulting fees from Novartis. PP has received consulting fees, speaking fees and/or honoraria from Novartis and SOBI. VH has received consulting fees, speaking fees and/or honoraria from Novartis, SOBI and MSD vaccin.
Figures
References
-
- Barron KS, Kastner DL. Periodic fever syndromes and other inherited autoinflammatory diseases. In: Petty RE, Laxer RM, Lindsey CB, Wedderburn LR, editors. Textbook of Pediatric Rheumatology. 7. Philadelphia: Elsevier; 2016. pp. 609–26.
-
- Georgin-Lavialle S, Rodriguez F, Hentgen V, Fayand A, Quartier P, Bader-Meunier, Bachmayer C, Savey L, Louvrier C, Sarrabay G, Melki I, Belot A, Koné-Pault I, Grateau G. Panorama Des maladies auto-inflammatoires. La Revue De Med Interne. 2018;39:240–55. doi: 10.1016/j.revmed.2018.02.005. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

