Exploring the potential of MB2 MBene family as promising anodes for Li-ion batteries
- PMID: 38590358
- PMCID: PMC10999910
- DOI: 10.1039/d4ra00287c
Exploring the potential of MB2 MBene family as promising anodes for Li-ion batteries
Abstract
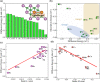
In recent years, finding high-performance energy storage materials has become a major challenge for Li-ion batteries. B-based two-dimensional materials have become the focus of attention because of their abundant reserves and non-toxic characteristics. A series of two-dimensional transition metal borides (MBenes) are reported and their electrochemical properties as anode materials for Li-ion batteries are investigated by density functional theory (DFT) calculations. The surface of MB2 possesses medium adsorption strength and diffusion energy barrier for Li atoms, which are conducive to the insertion and extraction of Li-ions during the charge/discharge process of Li-ion batteries. Herein, we explore the potential of MB2 (M = Sc, Y, Ti, Zr, Hf, V, Nb, Ta, Cr, Mo, W, Mn, Fe, Co, Ni, Cu and Zn) as the anode material for LIBs. Excitingly, the Li atom can be stably adsorbed on the surface of MB2 (M = Sc, Ti, V, Nb, Mo, W) monolayers, and the theoretical capacity of the MB2 monolayer is high (521.77-1610.20 mA h g-1). The average open circuit voltage range is within 0.10-1.00 V (vs. Li/Li+). The relationship between the p-band center of the B atom and the adsorption energy of Li on the surface of MB2 is also investigated. Furthermore, it is found that the charge transfer of Li atom and metallic center in the most stable position is strongly related to the corresponding value of diffusion energy barrier. These results confirm that MB2 monolayers are promising 2D anode materials for Li-ion batteries, demonstrating the application prospects of B-based 2D materials.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
-
- Liu J. Zhang J.-G. Yang Z. Lemmon J. P. Imhoff C. Graff G. L. Li L. Hu J. Wang C. Xiao J. Xia G. Viswanathan V. V. Baskaran S. Sprenkle V. Li X. Shao Y. Schwenzer B. Adv. Funct. Mater. 2013;23:929–946.
-
- Goriparti S. Miele E. De Angelis F. Di Fabrizio E. Proietti Zaccaria R. Capiglia C. J. Power Sources. 2014;257:421–443.
-
- Cheng X.-B. Zhang R. Zhao C.-Z. Zhang Q. Chem. Rev. 2017;117:10403–10473. - PubMed
-
- Tang Q. Zhou Z. Shen P. J. Am. Chem. Soc. 2012;134:16909–16916. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

