Duloxetine enhances the sensitivity of non-small cell lung cancer cells to EGFR inhibitors by REDD1-induced mTORC1/S6K1 suppression
- PMID: 38590408
- PMCID: PMC10998747
- DOI: 10.62347/WMQV6643
Duloxetine enhances the sensitivity of non-small cell lung cancer cells to EGFR inhibitors by REDD1-induced mTORC1/S6K1 suppression
Abstract
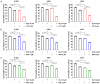
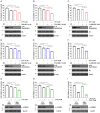
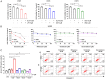
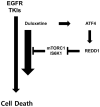
Although epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) have been effective targeted therapies for non-small cell lung cancer (NSCLC), most advanced NSCLC inevitably develop resistance to these therapies. Combination therapies emerge as valuable approach to preventing, delaying, or overcoming disease progression. Duloxetine, an antidepressant known as a serotonin-noradrenaline reuptake inhibitor, is commonly prescribed for the treatment of chemotherapy-induced peripheral neuropathy. In the present study, we investigated the combined effects of duloxetine and EGFR-TKIs and their possible mechanism in NSCLC cells. Compared with either monotherapy, the combination of duloxetine and EGFR-TKIs leads to synergistic cell death. Mechanistically, duloxetine suppresses 70-kDa ribosomal protein S6 kinase 1 (p70S6K1) activity through mechanistic target of rapamycin complex 1 (mTORC1), and this effect is associated with the synergistic induction of cell death of duloxetine combined with EGFR-TKIs. More importantly, activating transcription factor 4 (ATF4)-induced regulated in development and DNA damage response 1 (REDD1) is responsible for the suppression of mTORC1/S6K1 activation. Additionally, we found that the combination effect was significantly attenuated in REDD1 knockout NSCLC cells. Taken together, our findings reveal that the ATF4/REDD1/mTORC1/S6K1 signaling axis, as a novel mechanism, is responsible for the synergistic therapeutic effect of duloxetine with EGFR-TKIs. These results suggest that combining EGFR-TKIs with duloxetine appears to be a promising way to improve EGFR-TKI efficacy against NSCLC.
Keywords: ATF4; Duloxetine; EGFR-TKI; REDD1; S6K1; mTORC1; non-small cell lung cancer.
AJCR Copyright © 2024.
Conflict of interest statement
None.
Figures




References
-
- Sher T, Dy GK, Adjei AA. Small cell lung cancer. Mayo Clin Proc. 2008;83:355–367. - PubMed
-
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. - PubMed
-
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
