Hepatitis Delta Virus Reporting Requirements in the United States and Territories: A Systematic Review
- PMID: 38590737
- PMCID: PMC11000145
- DOI: 10.1093/ofid/ofae076
Hepatitis Delta Virus Reporting Requirements in the United States and Territories: A Systematic Review
Abstract
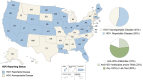
Hepatitis D virus (HDV) is a rare coinfection with hepatitis B virus. Currently, HDV is not a nationally notifiable disease in the United States. Only 55% of states and territories require HDV reporting, and most lack defined case definitions. Standardization of reporting requirements is crucial for monitoring HDV epidemiology.
Keywords: HDV; hepD; hepatitis D virus; hepatitis Delta virus; kolmioviridae.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. The authors have no conflicts of interest to declare.
Figures

References
-
- Shimkus J. R.4013–107th Congress (2001–2002): Rare Diseases Act of 2002. 2002. Accessed: February 25, 2024. Available at: https://www.congress.gov/bill/107th-congress/house-bill/4013.
-
- Centers for Disease Control and Prevention . Notifiable infectious disease tables. 2022. Available at: https://www.cdc.gov/nndss/data-statistics/infectious-tables/index.html.
-
- Wong RJ, Kaufman HW, Niles JK, et al. Low performance of hepatitis Delta virus testing among two national cohorts of chronic hepatitis B patients in the United States. Am J Gastroenterol 2022; 117:2067–70. - PubMed

