CCR2 and CCR5 co-inhibition modulates immunosuppressive myeloid milieu in glioma and synergizes with anti-PD-1 therapy
- PMID: 38590799
- PMCID: PMC11000615
- DOI: 10.1080/2162402X.2024.2338965
CCR2 and CCR5 co-inhibition modulates immunosuppressive myeloid milieu in glioma and synergizes with anti-PD-1 therapy
Abstract
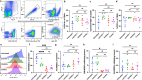
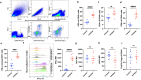
Immunotherapy has revolutionized the treatment of cancers. Reinvigorating lymphocytes with checkpoint blockade has become a cornerstone of immunotherapy for multiple tumor types, but the treatment of glioblastoma has not yet shown clinical efficacy. A major hurdle to treat GBM with checkpoint blockade is the high degree of myeloid-mediated immunosuppression in brain tumors that limits CD8 T-cell activity. A potential strategy to improve anti-tumor efficacy against glioma is to use myeloid-modulating agents to target immunosuppressive cells, such as myeloid-derived suppressor cells (MDSCs) in the tumor microenvironment. We found that the co-inhibition of the chemokine receptors CCR2 and CCR5 in murine model of glioma improves the survival and synergizes robustly with anti-PD-1 therapy. Moreover, the treatment specifically reduced the infiltration of monocytic-MDSCs (M-MDSCs) into brain tumors and increased lymphocyte abundance and cytokine secretion by tumor-infiltrating CD8 T cells. The depletion of T-cell subsets and myeloid cells abrogated the effects of CCR2 and CCR5 blockade, indicating that while broad depletion of myeloid cells does not improve survival, specific reduction in the infiltration of immunosuppressive myeloid cells, such as M-MDSCs, can boost the anti-tumor immune response of lymphocytes. Our study highlights the potential of CCR2/CCR5 co-inhibition in reducing myeloid-mediated immunosuppression in GBM patients.
Keywords: Glioma; Immunotherapy; MDSC.
© 2024 The Author(s). Published with license by Taylor & Francis Group, LLC.
Conflict of interest statement
M.L. has received research support from Arbor, BMS, Accuray, Tocagen, Biohaven, Kyrin-Kyowa and Biohaven, has been a consultant to Tocagen, VBI, InCephalo Therapeutics, Pyramid Bio, Merck, BMS, Insightec, Biohaven, Sanianoia, Hemispherian, Black Diamond Therapeutics, Novocure, is a shareholder of Egret Therapeutics and has patents for Focused radiation + checkpoint inhibitors, Local chemotherapy + checkpoint inhibitors and Checkpoint agonists for Neuro-Inflammation.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
