Design, synthesis and in silico molecular docking evaluation of novel 1,2,3-triazole derivatives as potent antimicrobial agents
- PMID: 38590856
- PMCID: PMC10999864
- DOI: 10.1016/j.heliyon.2024.e27773
Design, synthesis and in silico molecular docking evaluation of novel 1,2,3-triazole derivatives as potent antimicrobial agents
Abstract
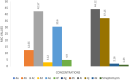
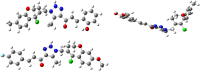
Chalcone and triazole scaffolds have demonstrated a crucial role in the advancement of science and technology. Due to their significance, research has proceeded on the design and development of novel benzooxepine connected to 1,2,3-triazolyl chalcone structures. The new chalcone derivatives produced by benzooxepine triazole methyl ketone 2 and different aromatic carbonyl compounds 3 are discussed in this paper. All prepared compounds have well-established structures to a variety of spectral approaches, including mass analysis, 1H NMR, 13C NMR, and IR. Among the tested compounds, hybrids 4c, 4d, 4i, and 4k exhibited exceptional antibacterial susceptibilities with MIC range of 3.59-10.30 μM against the tested S. aureus strain. Compounds 4c, 4d displayed superior antifungal activity against F. oxysporum with MIC 3.25, 4.89 μM, when compared to fluconazole (MIC = 3.83 μM) respectively. On the other hand, analogues 4d, 4f, and 4k demonstrated equivalent antitubercular action against H37Rv strain with MIC range of 2.16-4.90 μM. The capacity of ligand 4f to form a stable compound on the active site of CYP51 from M. tuberculosis (1EA1) was confirmed by docking studies using amino acids Leu321(A), Pro77(A), Phe83(A), Lys74(A), Tyr76(A), Ala73(A), Arg96(A), Thr80(A), Met79(A), His259(A), and Gln72(A). Additionally, the chalcone‒1,2,3‒triazole hybrids ADME (absorption, distribution, metabolism, and excretion), characteristics of molecules, estimations of toxicity, and bioactivity parameters were assessed.
Keywords: 1,2,3-Triazoles; ADMETlab2.0; Benzooxepines; Biological evaluations; Chalcones; Docking interactions.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Figures









References
-
- World Health Organization. 2021. https://www.who.int/news-room/fact-sheets/detail/tuberculosis accessed on 6th September, 2022.
-
- Global Tuberculosis Report 2020. World Health Organization; Geneva: 2020.
LinkOut - more resources
Full Text Sources
Research Materials

