Prenatal DEHP exposure induces lifelong testicular toxicity by continuously interfering with steroidogenic gene expression
- PMID: 38590960
- PMCID: PMC10999017
- DOI: 10.21037/tau-23-503
Prenatal DEHP exposure induces lifelong testicular toxicity by continuously interfering with steroidogenic gene expression
Abstract
Background: Epidemiologic studies suggested the association between prenatal di-(2-ethylhexyl) phthalate (DEHP) exposure and disorders of sex development (DSD), adult male disorders, and reproductive aging. Inhibiting testosterone synthesis by interfering with steroidogenic gene expression induces testicular toxicity, however, whether prenatal DEHP exposure induces testicular toxicity through this mechanism remains uncertain.
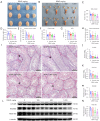
Methods: C57BL/6JGpt male mice underwent different doses (0, 100, 500, 1,000 mg/kg) of prenatal DEHP exposure during gestational day 10 to delivery day, the testicular toxicity (genital development, testosterone, semen quality, and morphology of testis tissue) in the neonatal, post-puberal and middle-aged stages was observed, and the steroidogenic gene (Lhcgr, Star, Cyp11a1, Cyp17a1, Hsd17b3, and Hsd3b2) expression was analyzed by quantitative polymerase chain reaction (qPCR) and Western blot (WB). The interference of steroidogenic gene expression in TM3 cells after mono-(2-ethylhexyl) phthalate (MEHP) exposure was also explored for verification.
Results: Prenatal DEHP exposure induced immediate testicular injury in the neonatal stage [reduced anogenital distance (AGD) and intratesticular testosterone], DSD in the post-puberal stage (poor genital development), and reproductive aging in the middle-aged stage (obesity, reduced testosterone and semen quality, and atrophic seminiferous tubules), especially in the high dose. Prenatal DEHP exposure continuously interfered with steroidogenic gene expression (Hsd3b2, Hsd17b3) in RNA and protein levels. MEHP inhibited testosterone synthesis of TM3 cells by interfering with steroidogenic gene expression (Hsd3b2, Hsd17b3) in RNA and protein levels.
Conclusions: Prenatal DEHP exposure induces lifelong testicular toxicity by continuously interfering with steroidogenic gene expression, thus indicating the association between prenatal exposure and DSD, adult male disorders, and reproductive aging.
Keywords: Disorders of sex development (DSD); di-(2-ethylhexyl) phthalate (DEHP); male disorders; sexual development; testis.
2024 Translational Andrology and Urology. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-503/coif). The authors have no conflicts of interest to declare.
Figures



Similar articles
-
Prenatal DEHP Exposure Induces Premature Testicular Aging by Promoting Leydig Cell Senescence through the MAPK Signaling Pathways.Adv Biol (Weinh). 2023 Oct;7(10):e2300130. doi: 10.1002/adbi.202300130. Epub 2023 May 28. Adv Biol (Weinh). 2023. PMID: 37246248
-
In utero exposure to di-(2-ethylhexyl) phthalate induces testicular effects in neonatal rats that are antagonized by genistein cotreatment.Biol Reprod. 2015 Oct;93(4):92. doi: 10.1095/biolreprod.115.129098. Epub 2015 Aug 26. Biol Reprod. 2015. PMID: 26316063 Free PMC article.
-
Exposure to DEHP and MEHP from hatching to adulthood causes reproductive dysfunction and endocrine disruption in marine medaka (Oryzias melastigma).Aquat Toxicol. 2014 Jan;146:115-26. doi: 10.1016/j.aquatox.2013.10.025. Epub 2013 Nov 8. Aquat Toxicol. 2014. PMID: 24292025
-
In utero exposure to di-(2-ethylhexyl) phthalate exerts both short-term and long-lasting suppressive effects on testosterone production in the rat.Biol Reprod. 2008 Jun;78(6):1018-28. doi: 10.1095/biolreprod.107.065649. Epub 2008 Mar 5. Biol Reprod. 2008. PMID: 18322279
-
Fetal origin of endocrine dysfunction in the adult: the phthalate model.J Steroid Biochem Mol Biol. 2013 Sep;137:5-17. doi: 10.1016/j.jsbmb.2013.01.007. Epub 2013 Jan 17. J Steroid Biochem Mol Biol. 2013. PMID: 23333934 Review.
References
LinkOut - more resources
Full Text Sources
