Atrial fibrillation symptom reduction and improved quality of life following the hybrid convergent procedure: a CONVERGE trial subanalysis
- PMID: 38590997
- PMCID: PMC10998972
- DOI: 10.21037/acs-2023-afm-15
Atrial fibrillation symptom reduction and improved quality of life following the hybrid convergent procedure: a CONVERGE trial subanalysis
Abstract
Background: CONVERGE was a prospective, multicenter, randomized controlled trial that evaluated the safety of Hybrid Atrial Fibrillation Convergent (HC) and compared its effectiveness to endocardial catheter ablation (CA) for the treatment of persistent atrial fibrillation (PersAF) and longstanding PersAF (LSPAF). In 2020, we reported that CONVERGE met its primary safety and effectiveness endpoints. The primary objective of the present study is to report CONVERGE trial results for quality of life (QOL) and Class I/III anti-arrhythmic drug (AAD) utilization following HC.
Methods: Eligible patients had drug-refractory symptomatic PersAF or LSPAF and a left atrium diameter ≤6.0 cm. Enrolled patients were randomized 2:1 to receive HC or CA. Atrial Fibrillation Severity Scale (AFSS) and the 36-Item Short Form Health Survey (SF-36) were assessed at baseline and 12 months; statistical comparison was performed using paired t-tests. AAD utilization at baseline through 12 and 18 months post-procedure was evaluated; statistical comparison was performed using McNemar's tests.
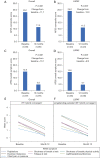
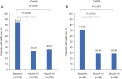
Results: A total of 153 patients were treated with either HC (n=102) or CA (n=51). Of the 102 HC patients, 38 had LSPAF. AFSS and SF-36 Mental and Physical Component scores were significantly improved at 12 months versus baseline with HC overall and for the subset of LSPAF patients treated with either HC or CA. The proportion of HC patients (n=102) who used Class I /III AADs at 12 and 18 months was significantly less (33.3% and 36.3%, respectively) than baseline (84.3%; P<0.001). In LSPAF patients who underwent HC (n=38), AADs use was 29.0% through 18 months follow-up versus 71.1% at baseline (P<0.001).
Conclusions: HC reduced AF symptoms, significantly improved QOL, and reduced AAD use in patients with PersAF and LSPAF.
Clinicaltrialsgov identifier: NCT01984346.
Keywords: Quality of life (QOL); anti-arrhythmic drug (AAD); atrial fibrillation (AF); catheter ablation (CA); hybrid convergent.
2024 Annals of Cardiothoracic Surgery. All rights reserved.
Conflict of interest statement
Conflicts of Interest: J.G. reports research funding from Abbott and lecture honoraria from AtriCure. C.B. has consulted for New Cardioplegia Solutions as well as proctoring for AtriCure. F.K. has received research grants from AtriCure; he has consulted for CryoLife, Edwards, LivaNova, and Medtronic. S.R.O. has consulted for Biosense Webster and has received compensation for services from AtriCure. M.A.M. has received compensation for services from AtriCure. M.E.H. reports advisory board membership and consultant fees from Medtronic. D.T. has received compensation for services from AtriCure. J.O. has consulted for Biosense Webster and Boston Scientific and has received compensation for services from AtriCure. S.A. reports speaker bureau membership for AtriCure. A.K. reports advisory board membership and consultant fees from AtriCure. C.S. reports consultant fees and honoraria from Abbott Laboratories, AtriCure, and Medtronic. D.M.G. has received compensation for services from AtriCure. M.K. reports consultant fees from Medtronic. I.J. reports advisory board membership and consultant fees from AtriCure. F.Y. reports advisory board membership and consultant fees from AtriCure. D.B.D.L. is a consultant and speaker for AtriCure and Boston Scientific, and a consultant to Medtronic. The other authors have no conflicts of interest to declare.
Figures


References
-
- Charitakis E, Barmano N, Walfridsson U, et al. Factors Predicting Arrhythmia-Related Symptoms and Health-Related Quality of Life in Patients Referred for Radiofrequency Ablation of Atrial Fibrillation: An Observational Study (the SMURF Study). JACC Clin Electrophysiol 2017;3:494-502. 10.1016/j.jacep.2016.12.004 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
