Unveiling the diversity, composition, and dynamics of phyllosphere microbial communities in Alhagi sparsifolia across desert basins and seasons in Xinjiang, China
- PMID: 38591034
- PMCID: PMC10999668
- DOI: 10.3389/fmicb.2024.1361756
Unveiling the diversity, composition, and dynamics of phyllosphere microbial communities in Alhagi sparsifolia across desert basins and seasons in Xinjiang, China
Abstract
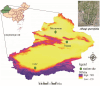
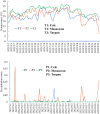
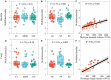
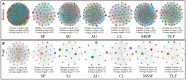
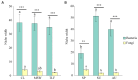
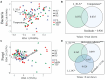
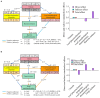
Phyllosphere microbes residing on plant leaf surfaces for maintaining plant health have gained increasing recognition. However, in desert ecosystems, knowledge about the variety, composition, and coexistence patterns of microbial communities in the phyllosphere remains limited. This study, conducted across three basins (Turpan-TLF, Tarim-CL, and Dzungaria-MSW) and three seasons (spring, summer, and autumn) in Xinjiang, China, aimed to explore the diversity and composition of microbial communities in the phyllosphere, encompassing both bacteria and fungi in Alhagi sparsifolia. We also investigated the co-occurrence patterns, influencing factors, and underlying mechanisms driving these dynamics. Results indicate that phyllosphere bacteria exhibited lower diversity indices (ACE, Shannon, Simpson, Fisher phylogenetic diversity, and Richness) in spring compared to summer and autumn, while the Goods Coverage Index (GCI) was higher in spring. Conversely, diversity indices and GCI of phyllosphere fungi showed an opposite trend. Interestingly, the lowest level of multi-functionality and niche width in phyllosphere bacteria occurred in spring, while the highest level was observed in phyllosphere fungi. Furthermore, the study revealed that no significant differences in multi-functionality were found among the regions (CL, MSW, and TLF). Network analysis highlighted that during spring, phyllosphere bacteria exhibited the lowest number of nodes, edges, and average degree, while phyllosphere fungi had the highest. Surprisingly, the multi-functionality of both phyllosphere bacteria and fungi showed no significant correlation with climatic and environmental factors but displayed a significant association with the morphological characteristics and physicochemical properties of leaves. Structural Equation Model indicated that the morphological characteristics of leaves significantly influenced the multi-functionality of phyllosphere bacteria and fungi. However, the indirect and total effects of climate on multi-functionality were greater than the effects of physicochemical properties and morphological characteristics of leaves. These findings offer new insights into leaf phyllosphere microbial community structure, laying a theoretical foundation for vegetation restoration and rational plant resource utilization in desert ecosystems.
Keywords: climate effects; co-occurrence patterns; desert ecosystems; phyllosphere microbiome; seasonal dynamics.
Copyright © 2024 Zhang, Du, Zhang, Islam and Zeng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Variation in Root-Associated Microbial Communities among Three Different Plant Species in Natural Desert Ecosystem.Plants (Basel). 2024 Sep 3;13(17):2468. doi: 10.3390/plants13172468. Plants (Basel). 2024. PMID: 39273952 Free PMC article.
-
Summer season and long-term drought increase the richness of bacteria and fungi in the foliar phyllosphere of Quercus ilex in a mixed Mediterranean forest.Plant Biol (Stuttg). 2012 Jul;14(4):565-75. doi: 10.1111/j.1438-8677.2011.00532.x. Epub 2012 Jan 30. Plant Biol (Stuttg). 2012. PMID: 22289059
-
Plant species shape the bacterial communities on the phyllosphere in a hyper-arid desert.Microbiol Res. 2023 Apr;269:127314. doi: 10.1016/j.micres.2023.127314. Epub 2023 Jan 27. Microbiol Res. 2023. PMID: 36724560
-
Affecting Factors of Plant Phyllosphere Microbial Community and Their Responses to Climatic Warming-A Review.Plants (Basel). 2023 Aug 8;12(16):2891. doi: 10.3390/plants12162891. Plants (Basel). 2023. PMID: 37631103 Free PMC article. Review.
-
Phyllosphere microbiology with special reference to diversity and plant genotype.J Appl Microbiol. 2008 Dec;105(6):1744-55. doi: 10.1111/j.1365-2672.2008.03906.x. J Appl Microbiol. 2008. PMID: 19120625 Review.
Cited by
-
Variation in Root-Associated Microbial Communities among Three Different Plant Species in Natural Desert Ecosystem.Plants (Basel). 2024 Sep 3;13(17):2468. doi: 10.3390/plants13172468. Plants (Basel). 2024. PMID: 39273952 Free PMC article.
-
Impact of seasonal changes on root-associated microbial communities among phreatophytes of three basins in desert ecosystem.Front Plant Sci. 2025 Jul 4;16:1554879. doi: 10.3389/fpls.2025.1554879. eCollection 2025. Front Plant Sci. 2025. PMID: 40688685 Free PMC article.
References
-
- Bao S. D. (2000). Analysis of soil and Agrochemistry, 3rd Edn. Beijing: China Agriculture Press.
-
- Benjamini Y., Krieger A. M., Yekutieli D. (2006). Adaptive linear step-up procedures that control the false discovery rate. Biometrika 93, 491–507. doi: 10.1093/biomet/93.3.491 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

