Bispecific VEGF-A and Angiopoietin-2 Antagonist RO-101 Preclinical Efficacy in Model of Neovascular Eye Disease
- PMID: 38591047
- PMCID: PMC11000112
- DOI: 10.1016/j.xops.2024.100467
Bispecific VEGF-A and Angiopoietin-2 Antagonist RO-101 Preclinical Efficacy in Model of Neovascular Eye Disease
Abstract
Objective: To investigate preclinical data regarding the efficacy and biocompatibility of a bispecific protein, RO-101, with effects on VEGF-A and angiopoietin-2 (Ang-2) for use in retinal diseases.
Design: Experimental study.
Subjects: Brown Norway rats and New Zealand White Cross rabbits.
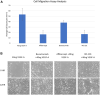
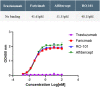
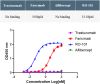
Methods: Preclinical study data of RO-101 in terms of target-specific enzyme-linked immunosorbent assay binding affinity to VEGF-A and Ang-2, vitreous half-life, inhibition of target-receptor interaction, laser choroidal neovascular membrane animal model, human umbilical vein endothelial cell migration, and biocompatibility was obtained. Where applicable, study data were compared with other anti-VEGF agents.
Main outcome measures: Binding affinity, half-life, biocompatibility, and efficacy of RO-101. Neovascularization prevention by RO-101.
Results: RO-101 demonstrated a strong binding affinity for VEGF-A and Ang-2 and in vitro was able to inhibit binding to the receptor with higher affinity than faricimab. The half-life of RO-101 is comparable to or longer than current VEGF inhibitors used in retinal disease. RO-101 was found to be biocompatible with retinal tissue in Brown Norway rats. RO-101 was as effective or more effective than current anti-VEGF therapeutics in causing regression of neovascular growth in vivo.
Conclusions: RO-101 is a promising candidate for use in retinal diseases. In preclinical models, RO-101 demonstrated similar or higher regression of neovascular growth to current anti-VEGF therapeutics with comparable or longer half-life. It also demonstrates a strong binding affinity for VEGF-A and Ang-2. It also was shown to be biocompatible with retinal tissue in animal studies, indicating potential compatibility for use in humans.
Financial disclosures: Proprietary or commercial disclosure may be found in the Footnotes and Disclosures at the end of this article.
Keywords: AMD; Ang-2; Anti-VEGF; Bispecific; Surrobody.
© 2024 by the American Academy of Ophthalmology.
Figures







References
-
- Kvanta A., Algvere P.V., Berglin L., Seregard S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci. 1996;37:1929–1934. - PubMed
-
- Lopez P.F., Sippy B.D., Lambert H.M., Thach A.B., Hinton D.R. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1996;37:855–868. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

